If you’re coming from our “Bonding and Chemical Reactions” chapter overview in our general chemistry module, this chapter overview might seem like a refresher of that other overview and in a way, it is!
When you think of organic chemistry, perhaps images of arrow drawing and electron pairs come to mind; however, keep in mind that the ultimate result of these processes is either the formation or breaking of bonds!
This chapter overview will cover some of the basics in bonding in the context of organic chemistry. Let’s get started!
Bonding on the MCAT: What You Need to Know
Topics on organic chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
Luckily, this section is not as heavily focused or tested on compared to other topics! Questions covering bonding are a bit more vague as we’ll get into shortly.
Introductory organic chemistry accounts for 15% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems
Important Sub-Topics: Bonding
As mentioned above, questions on bonding are a bit more obscure as the MCAT probably won’t test questions covering organic chemistry bonding directly; rather, they’ll expect you to apply the concepts learned here to passage content!
Similarly, the content covered in this chapter overview and the individual articles will also serve as a good basis for understanding the organic chemistry reactions involving nucleophiles and electrophiles.
1. Review of Dipoles and Polarity
Let’s first start with a general review of dipole moments and the charge polarization generated by them! Recall that a dipole occurs between 2 bonded atoms that differ in electronegativity.
This difference in their electronegativities generates an uneven distribution of electrons where the more electronegative atom “attracts” the electrons within the bond! As a result, one atom has a positive dipole while the other has a negative dipole.
A common textbook example is water: within the water molecule, the oxygen atom has a higher electronegativity than the hydrogen atoms and “pulls” the negatively charged electrons towards itself, which results in its overall negative dipole. Because the hydrogens have the electrons “stripped away”, they retain a positive dipole!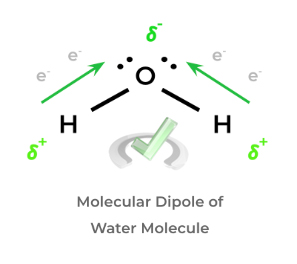
As shown, this dipole generates a polarization of charge in the water molecule. While topics on molecular dipoles and polarity are usually placed in the context of intermolecular forces, they too have important implications in organic chemistry reactions, as we’ll cover in the next section!
Full Study Notes : Dipoles and Polarities
For more in-depth discussion of dipoles and polarities as well as our other chapter overview and articles located in the general chemistry module, check out these detailed lesson notes created by top MCAT scorers.
2. Dipoles and Polarity in Nucleophilic Additions/Substitutions
To understand the implications of dipoles and polarity in nucleophilic reactions with electrophilic species, let’s first differentiate between nucleophilic and electrophilic molecules!
Nucleophilic molecules are electron rich molecules that are attracted to the overall positive charge to an electron poor molecule. Conversely, electrophilic molecules are electron poor molecules that can be targeted by nucleophiles – as such, most electrophiles are usually positively charged.
Note that nucleophiles and electrophiles can also be defined as Lewis bases and acids, respectively, via the ability to donate or accept electron pairs; however, we wanted to include their general charges to connect with the topic of dipoles and polarity.
These ideas are important as dipoles within a molecule can cause a polarity of charge. As such, polarization of charge can cause one portion of the molecule to have a positive charge which, in most cases, allows it to function as an electrophile and be targeted by a nucleophile! Let’s look at the most high yield example: the carbonyl group.
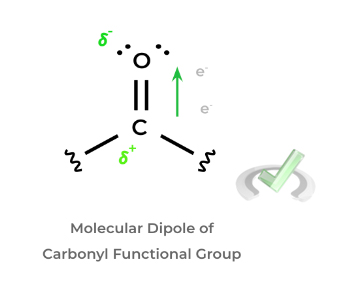
As shown, the dipole created by the carbonyl functional group results in a charge polarization where the carbon atom gains a positive dipole. This also gives the carbonyl carbon electrophilic character to where it can be targeted by a nucleophile!
Shown below is the esterification (formation of an ester) from a carboxylic acid and an alcohol. Though not all the steps and products are shown, note the importance of the alcohol’s nucleophilic targeting on the electrophilic carbonyl carbon!
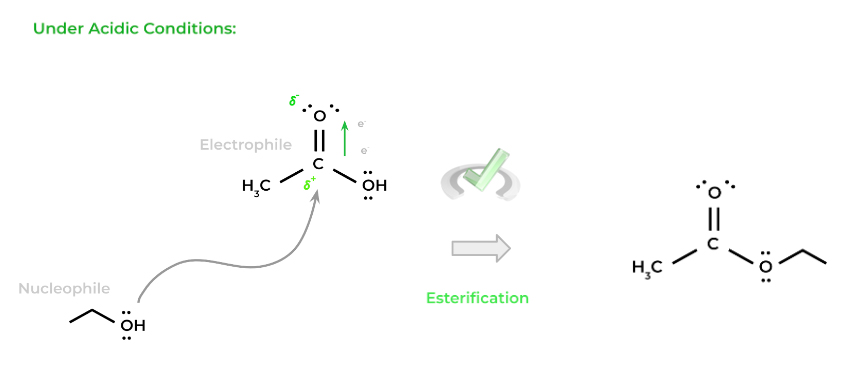
Full Study Notes : Organic nucleophilic additions and substitution reactions
For more in-depth discussion of the importance of discussing dipoles and polarity in regard to organic nucleophilic additions and substitution reactions is a little more clear, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about organic chemistry bonding!
Term | Definition |
|---|---|
Dipole | The unequal distribution of electrons generated by 2 bonded atoms with differing electronegativities |
Polarity | Refers to the separation of charge within a molecule |
Nucleophile | An electron rich species which is attracted to the overall positive charge of a electron poor species; also defined as a Lewis base due to its ability to donate an electron pair |
Electrophile | An electron poor species – generally positive – which can be targeted by a nucleophile; also defined as a Lewis acid due to its ability to accept an electron pair |
Additional Reading Links – Study Notes for Bonding on the MCAT
Additional Reading -- Organic Chemistry Topics:
- Alcohols and Ethers on the MCAT
- Aldehydes and Ketones on the MCAT
- Carboxylic Acids and Derivatives on the MCAT
- Isomers on the MCAT
- Laboratory Techniques and Separations on the MCAT
- Nitrogen Containing Compounds on the MCAT
- Phosphorus Containing Compounds on the MCAT
- Organic Chemistry Nomenclature on the MCAT
- Nucleophiles and Electrophiles on the MCAT
- Spectroscopy on the MCAT
- Redox Reactions Organic Chemistry on the MCAT







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
