We’ve discussed a lot about nucleophilic attack and how this kickstarts important chemical reactions involving carbonyl groups. This nucleophilic attack starts a series of bond changes that ultimately form complex compounds such as carboxylic acid derivatives! Before we get more into how dipoles and polarity take part in nucleophilic attack, let’s first look at the carbonyl group.
I. Polarity
As we’ve discussed earlier, the polarity of a compound is a result of the formation of positive and negative poles in a molecule. A positive pole lacks electrons. Electrons are the ones that carry negative charges, as such, when there is a lack of electrons, it creates a positive pole.
A negative pole, on the other hand, is a region with a surplus of electrons. Because it’s electron-rich, the overall charge of a pole becomes negative.
However, we do not use the term positive or negative when we refer to poles. This is to not give the notion that this positivity or negativity is a “permanent” feature. The creation of a dipole moment is merely a result of the surplus or lack of electrons. The distribution of electrons is not permanent, because in a polar molecule, one atom tends to pull more electrons than the other, it creates a dipole moment where one side is positive and another is negative.
We call a positive pole “partially positive” and a negative pole “partially negative.” A carbonyl group has a central carbon atom with a double bond with oxygen. The same carbon atom may be bonded to other stuff like a carbon chain or an OH group–it really doesn’t matter.
What we should focus on is the property that makes carbonyl groups highly efficient in creating complex derivatives.
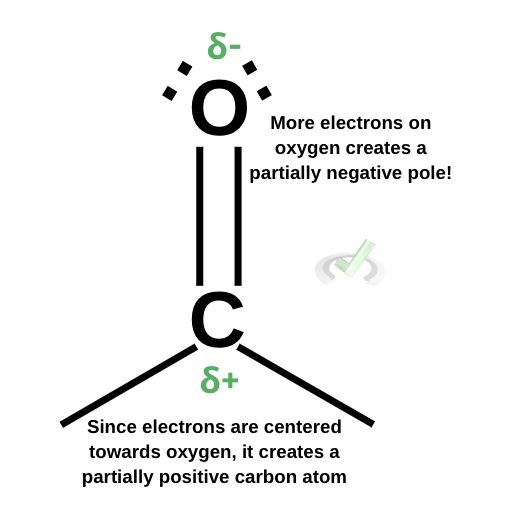
A carbonyl has a partially negative pole on oxygen because all of the electrons are centered around it. Oxygen also has a greater electronegativity (3.44) than carbon (2.55). Because of this, electrons tend to move towards oxygen. Remember that electronegativity is a property that shows how likely it is for an atom to attract an electron.
This makes all of the electrons center around where the oxygen atom is. Because oxygen tends to have a greater “pull” towards electrons, this creates a partially positive carbon atom. The lack of electrons also means that carbon will essentially lack negative charges. This makes for a partially positive carbon atom. This partial positivity makes it an electrophile because it lacks negative charges from electrons, making it bear a partially positive charge.II. Nucleophilic Attack
Now that we’ve established that carbonyl groups have a partially negative oxygen and a partially positive carbon, thereby making it an electrophile, let’s see how this relates to nucleophilic attack. As discussed in our previous articles, carboxylic acid derivatives undergo nucleophilic attack. Since a carbonyl group has a partially positive carbon, making it an electrophile, it becomes susceptible to a nucleophilic attack.
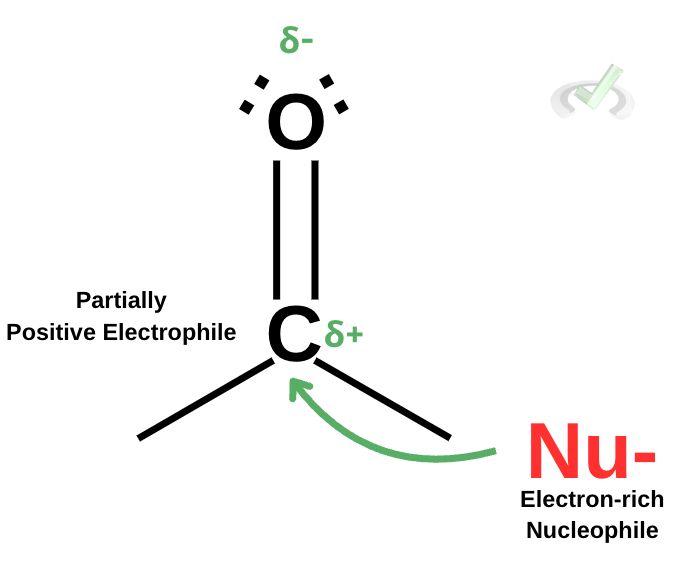
We use the term nucleophilic attack to merely point out that it (nucleophile, being electron-rich) somehow “attacks” an electrophile that is (electron-deficient). Nucleophiles are chemical species that are rich in electrons. When it attacks an electrophile such as a carbonyl carbon, the nucleophile donates electrons to the electron-deficient carbonyl carbon to form a bond.
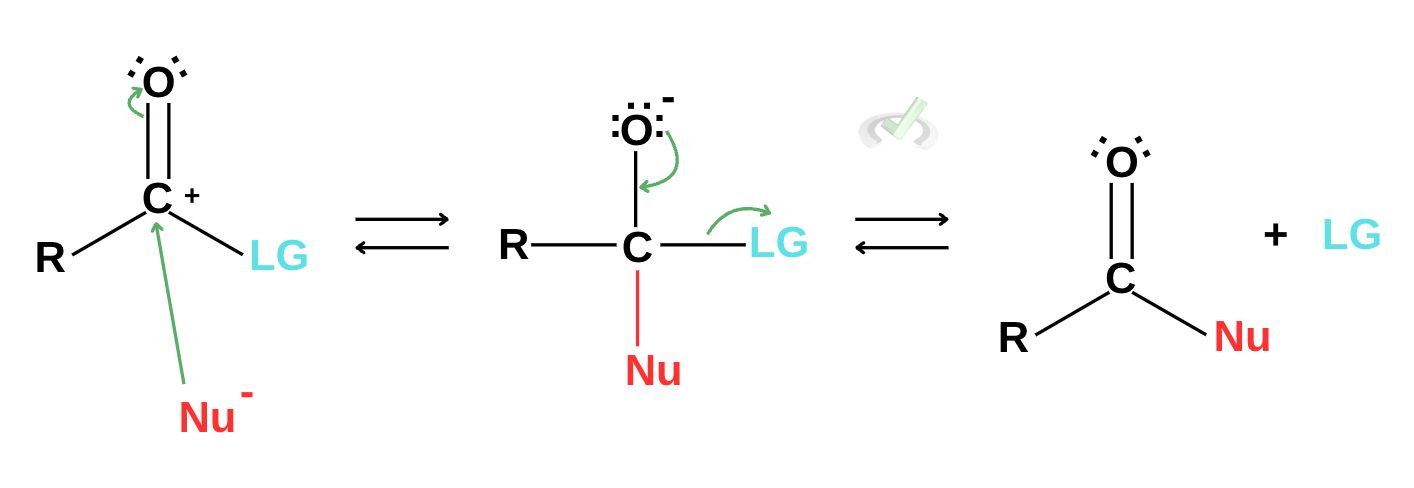
The reaction above shows the mechanism of nucleophilic substitution reactions of carboxylic acids. This substitution reaction begins when a nucleophile is attracted to an electrophile. This creates a bond between the nucleophile and the carbon. This also causes a series of rearrangement of electrons and bonds that result in the formation of a carboxylic acid derivative with the nucleophile and the ejection of a leaving group.
III. Conclusion
Carbonyl groups are involved in the formation of complex derivatives due to their polarity. This polarity is caused by the distribution of electrons around the group. The oxygen atom, being more electronegative, pulls more electrons toward itself, creating a partially negative oxygen since electrons have negative charge. As a result, carbon, being less electronegative, bears a partially positive charge. This makes the carbonyl carbon an electrophile. Nucleophiles are chemical species that have electrons to donate. When a nucleophile attacks an electrophile, it donates its electrons to form a bond with the carbonyl carbon.
IV. Key Terms
- Carbonyl carbon - the central carbon atom of a carbonyl group
- Electrophile - an electron-deficient chemical species that bears a partially positive charge.
- Nucleophile - an electron-rich chemical species that bears a partially negative charge.
IV. Practice Problems
Use the image below to answer questions 1 and 2.
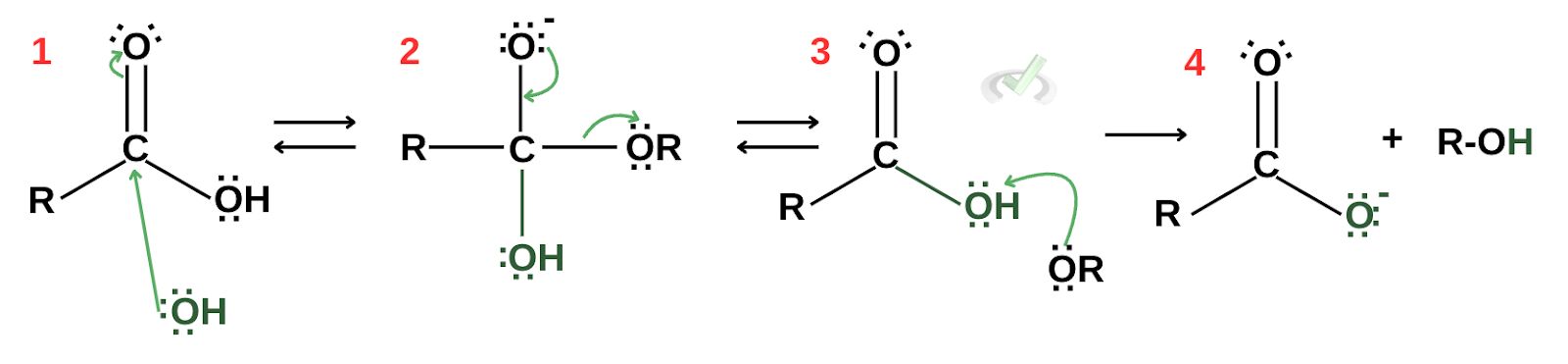
Sample Practice Question 1
In the given reaction, in which step does nucleophilic attack occur?
A. Step 1
B. Step 2
C. Step 3
D. Step 4
Ans. A
Sample Practice Question 2
Which of the following is the nucleophile in the reaction?
A. R-C-OH
B. COOH
C. R-OH
D. OH
Ans. D







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
