Naming different configurations in organic chemistry is vital to understanding molecular structures. This guide covers the basics and complexities of configuration naming, providing a foundation for more advanced topics in organic chemistry.
I. Basics of Configuration Naming
A. What is Configuration?
Configuration is the term used to define spatial arrangement of atoms in a molecule. Even if the molecular formula is the same, different configurations can lead to different chemical properties, such as boiling point, solubility, and reactivity.
B. Why is Configuration Important?
Knowing the configuration helps predict the behavior of molecules in chemical reactions. It also aids in understanding biological activity and the physical properties of compounds. For example, the efficacy of a drug can depend on its configuration.
II. Types of Configurations
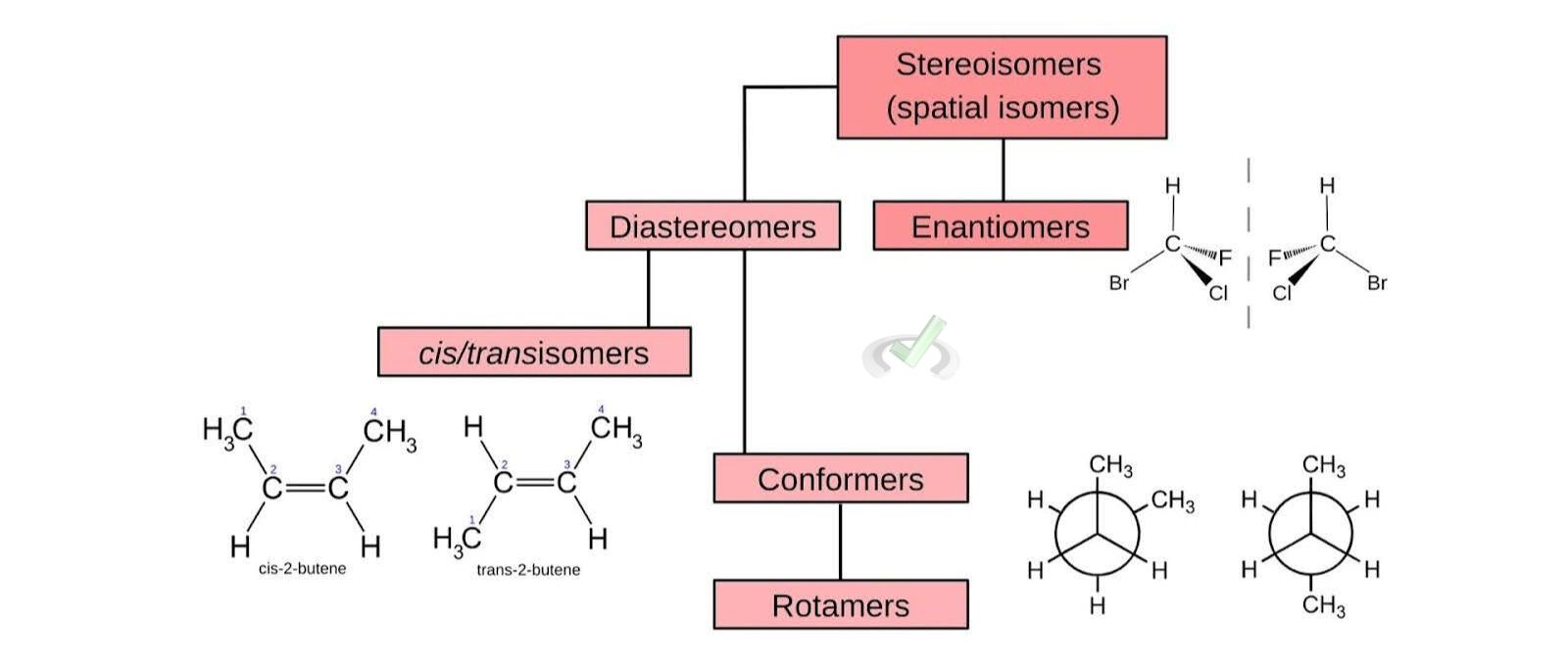
A. Stereoisomers
Stereoisomers are compounds with the same molecular formula and sequence of bonded atoms but differ in the three-dimensional orientations of their atoms.
- Enantiomers: These are non-superimposable mirror images of each other. Think of your left and right hands. They are similar but not identical. An example is the pair of D- and L-glucose.
- Diastereomers: These are stereoisomers that are not mirror images of each other. They have different physical properties and reactivity. For instance, cis and trans isomers in alkenes.
- Conformers (Conformational Isomers): These are stereoisomers that differ by rotation around a single bond. They can interconvert by rotating about the bond. For example, the different staggered and eclipsed forms of ethane.
- Rotamers: These are a type of conformational isomer where the rotation occurs around a single bond, leading to different spatial arrangements. For instance, the different spatial arrangements of atoms in butane due to rotation around the C-C bond.
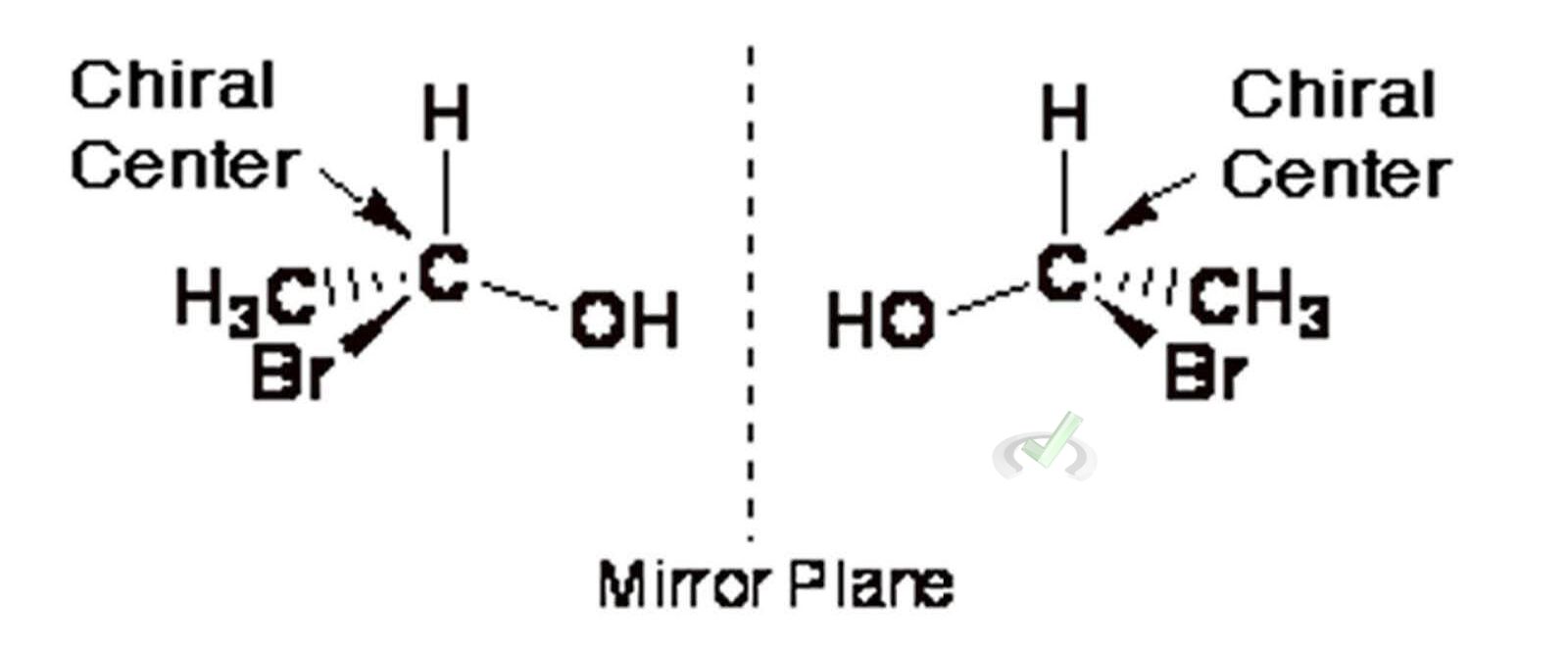
B. Chiral Centers
A chiral center (or stereocenter) is a carbon atom bonded to four different groups. This causes the molecule to have non-superimposable mirror images (enantiomers).
Example: Lactic acid has a chiral center.
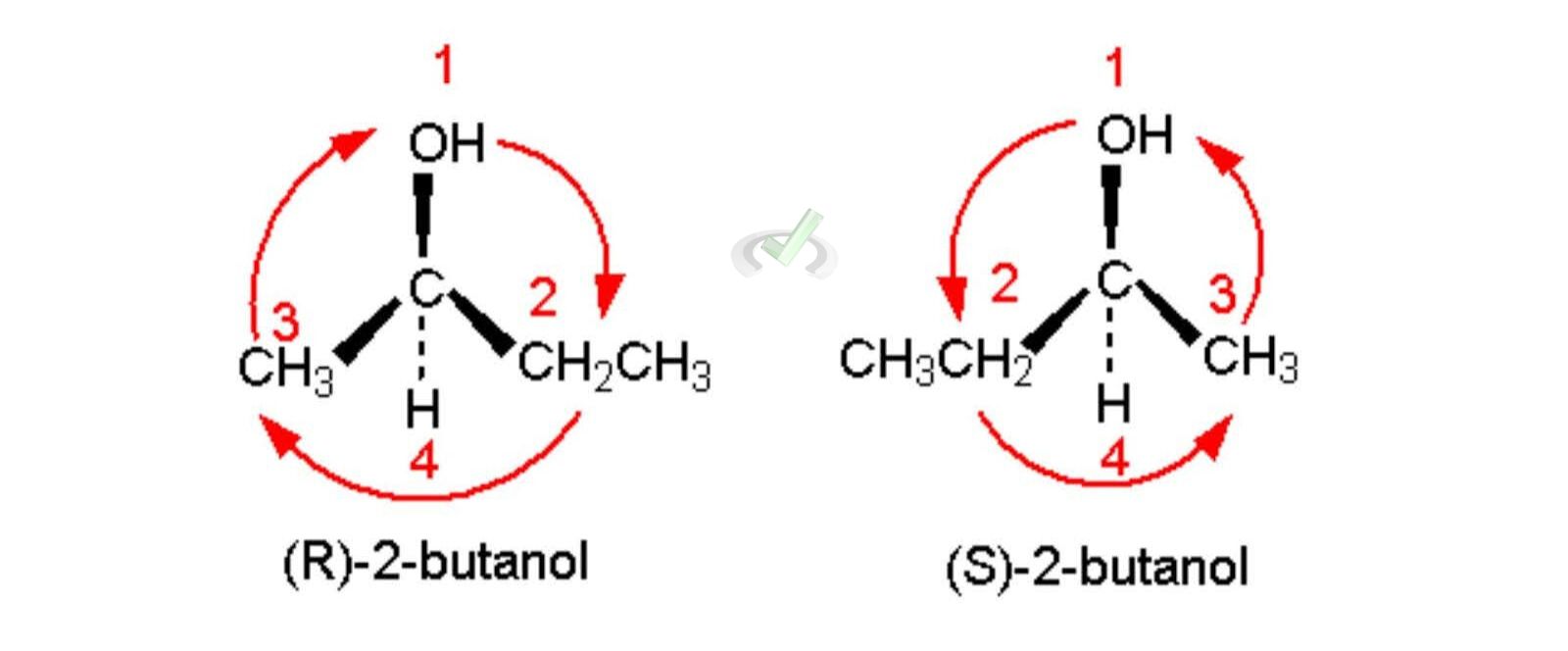
C. Cahn-Ingold-Prelog Priority Rules
These rules determine the priority of groups attached to a chiral center.
- Assign Priorities: Based on atomic number. Higher atomic numbers get higher priority.
- Orient the Molecule: The group with the lowest priority (usually hydrogen) should be oriented away from the viewer.
- Determine the Configuration: If the sequence 1-2-3 is clockwise, it is R (rectus); if counterclockwise, it is S (sinister).
Example: Determining R and S configuration for 2-butanol (CH3CH(OH)CH2CH3).
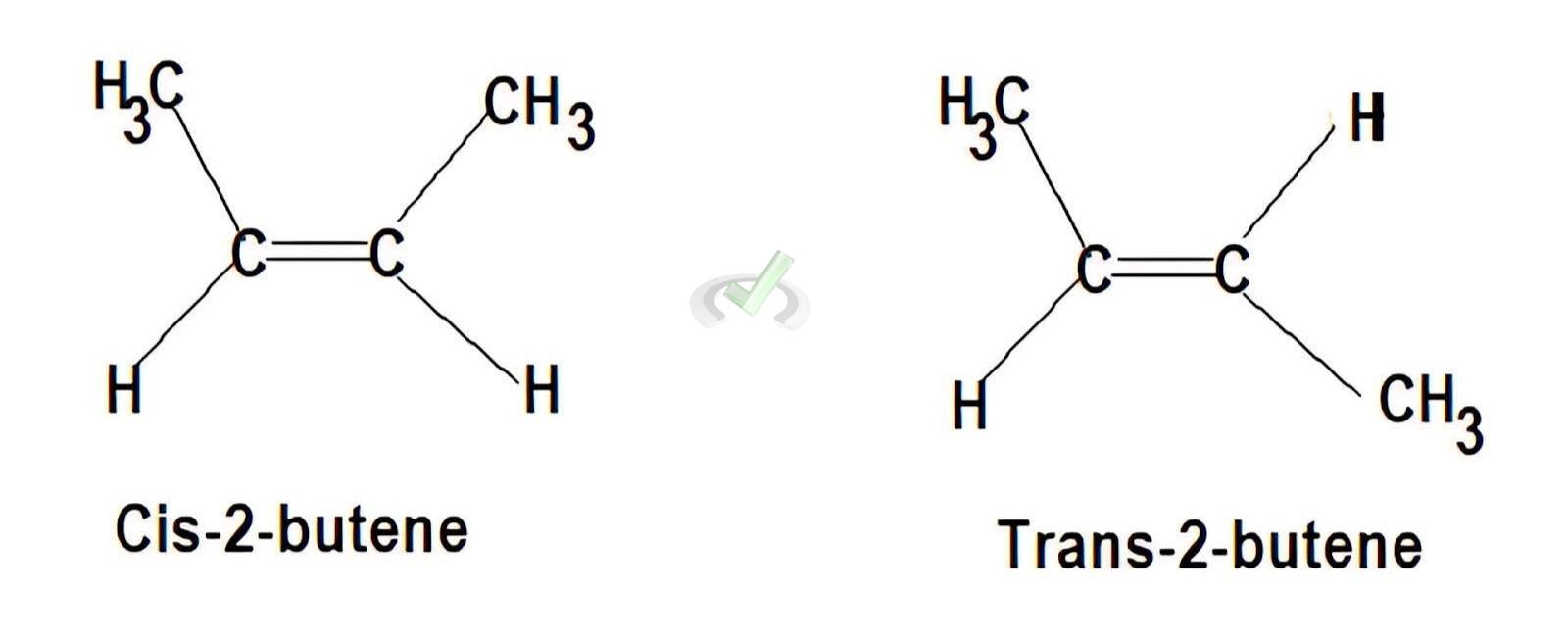
III. Geometric Isomers
A. Cis-Trans Isomerism
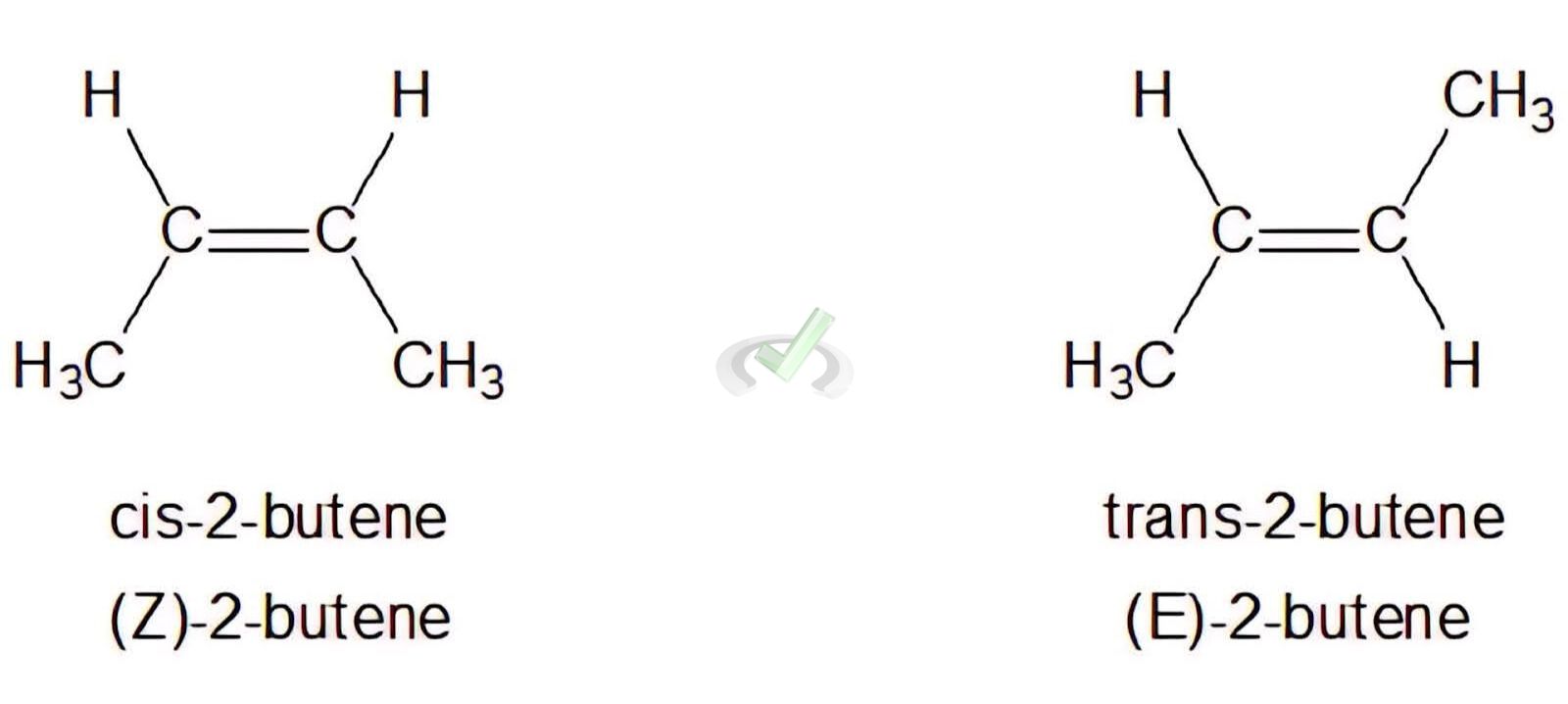
Cis-trans isomerism occurs in alkenes where groups can be on the same side (cis) or opposite sides (trans) of the double bond.
B. E-Z Notation
For alkenes with more complex substituents, the E-Z notation is used.
- Assign Priorities: Similar to Cahn-Ingold-Prelog rules.
- Identify Positions: If the higher priority groups are on the same side, it is Z (zusammen); if on opposite sides, it is E (entgegen).
Example: Determining E-Z configuration for 2-butene (CH3CH=CHCH3).
IV. Optical Activity
A. Stereochemistry in Reactions
Stereochemistry plays a critical role in the outcome of chemical reactions. For example, in nucleophilic substitution reactions (SN1 and SN2), the arrangement of atoms affects the reaction mechanism and the final product. Understanding stereochemistry helps predict which product will form.
B. Biological Implications
Chirality is crucial in biological systems. For instance, only one enantiomer of a drug may be active. The other enantiomer might be less effective or even harmful. This is important for pharmacology, as it helps explain why certain drugs work the way they do.
C. Advanced Topics
Understanding configuration naming lays the groundwork for studying reaction mechanisms and the synthesis of complex molecules. This knowledge is essential for organic synthesis, which aims to create specific molecules with desired configurations. This links to organic synthesis and reaction mechanism studies.
D. Enzyme Catalysis
Many enzymes are chiral and work by recognizing the specific configuration of molecules. This is important for understanding enzyme mechanisms and drug design. Knowing how enzymes interact with different configurations can help in developing new medications.
VI. Wrap-Up and Key Terms
Understanding the naming of different configurations is essential for grasping more complex topics in organic chemistry. It involves recognizing stereoisomers, applying priority rules, and identifying optical activity. This foundational knowledge is crucial for studying chemical reactions and biological processes.
Key Terms
- Stereoisomer: Molecules with the same molecular formula but different spatial arrangements.
- Enantiomer: Non-superimposable mirror images.
- Diastereomer: Stereoisomers that are not mirror images.
- Chiral Center: A carbon atom that is bonded to four different groups.
- Cis-Trans Isomerism: Different arrangements of groups around a double bond.
- E-Z Notation: Describes the relative positions of groups around a double bond.
- Optical Activity: The rotation of plane-polarized light by chiral molecules.
VII. Practice Questions
Sample Practice Question 1
What is the R/S configuration of 2-butanol?
A) R
B) S
C) Both R and S
D) Neither R nor S
Ans. A
The configuration of 2-butanol is R. This is determined by assigning priorities based on atomic numbers and orienting the lowest priority group away from the viewer. The sequence from highest to lowest priority in a clockwise direction confirms it is R.
Sample Practice Question 2
How do you determine if two molecules are enantiomers?
A) Check if they are mirror images.
B) Check if they have the same molecular formula.
C) Check if they have the same sequence of bonded atoms.
D) All of the above
Ans. A
Enantiomers are defined as non-superimposable mirror images. This means they have the same molecular formula and sequence of bonded atoms. However, they differ in their three-dimensional arrangement, specifically being mirror images.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
