Amino acids serve as the building blocks of proteins. Proteins regulate many reactions in our body; from building up muscle tissue to help us move to allowing our cells to receive oxygen. It’s essential to master the basics of amino acids as this topic will continue to haunt you in your future life in med school. In this lesson, we’ll learn more about what amino acids are, their structure, and their role in our DNAs.
I. Structure
Amino acids have the same basic structures. They have an amino group (NH₂) attached on one side, a carboxyl group (-COOH) on the other, and a side chain (R). The carbon that connects these three is an alpha carbon since it’s adjacent to a carbonyl carbon.
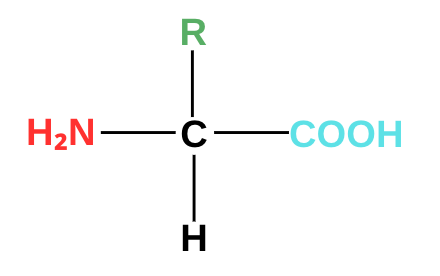
Amino group (NH₂): This group includes a nitrogen bonded to two hydrogen atoms. This group is basic in nature. It serves as the site for the formation of peptide bonds. Peptide bonds are bonds that connect amino acids together to form proteins.
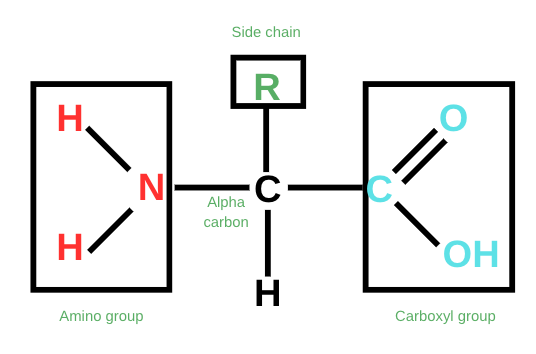
Carboxyl Group (-COOH): Consists of a carbon double bonded to oxygen and a single bong with a hydroxyl (-OH) group. This group is acidic and can deprotonate to form peptide bonds. In peptide bonding, a carboxyl group of one amino acid forms a bond with an amino group of another amino acid, forming the backbone of a protein chain.
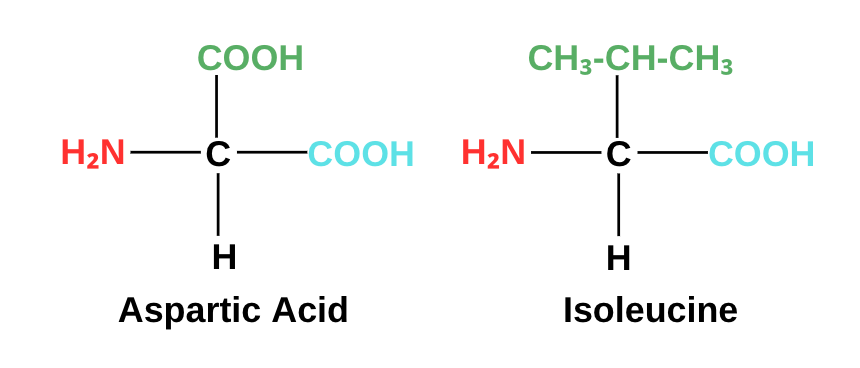
Side chain (R): Provides the character for the amino acid. This group is what distinguishes one amino acid from another. Aspartic acid, for instance, is different from isoleucine because its side chains are different. Our body uses about 20 standard amino acids to carry out its basic functions. These amino acids play an important role in biological systems, as we will find out later.
II. Peptide Bonds
Peptide bonds form when a carboxyl group of one amino acid establishes a covalent bond with an amino group of another chain. The molecules that form when amino acids join are known as peptides; the amide linkages are called peptide bonds. Peptides may have two or many amino acids. They are dipeptides when there are two amino acids, tripeptides for three amino acids, oligopeptides for four to twenty amino acids, and polypeptides for more than twenty amino acids.
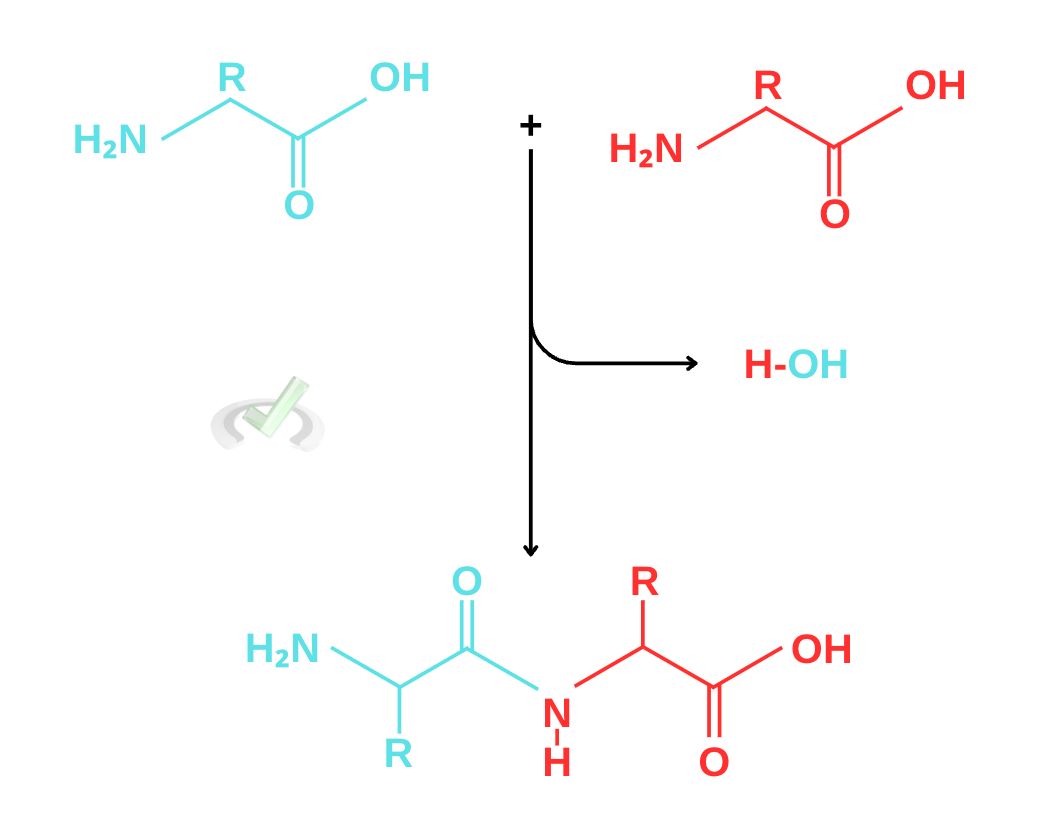
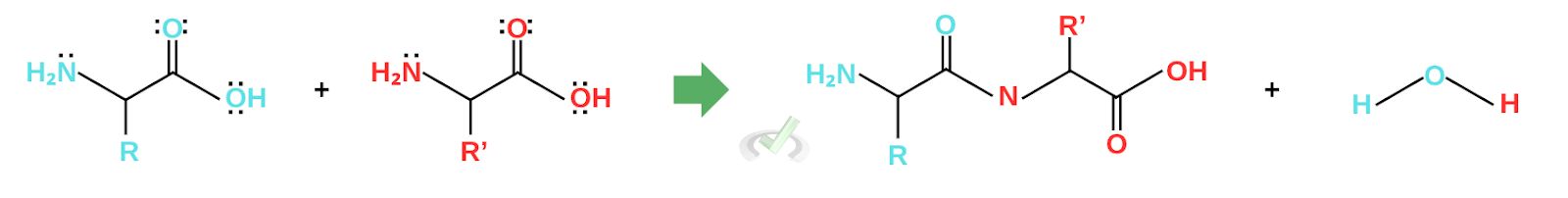
The mechanism for peptide bond formation is also a condensation reaction and uses the same nucleophilic substitution mechanism. The amino group from another amino acid acts as a nucleophile and initiates nucleophilic addition by attacking the carbonyl carbon. This changes the rearrangement of electrons, forming an intermediate. The electrons continue to rearrange to gain a neutral charge until they eject water as a leaving group and form the peptide. This reaction is also called a condensation reaction since it produces water at the end of the reaction.
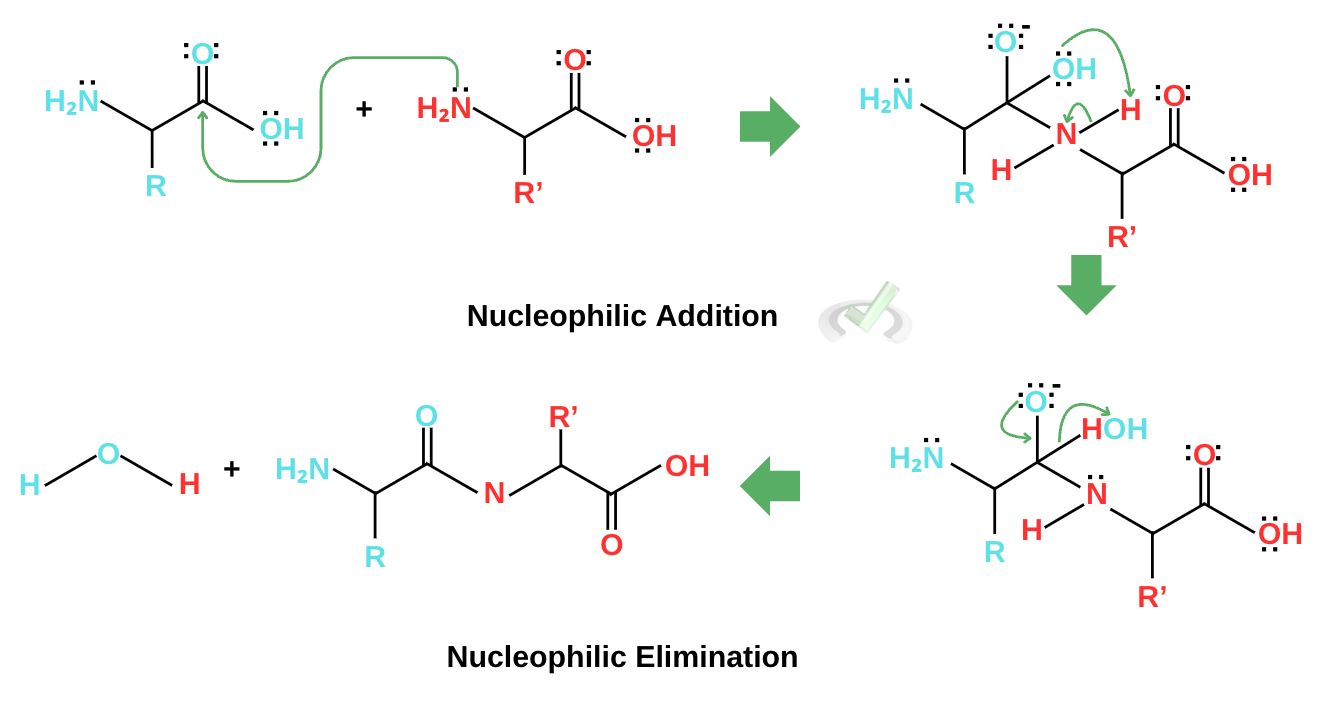
Nitrogen is essential in peptide bond formation since it acts as a nucleophile and attaches itself to the carboxyl group of another amino acid. This mechanism lets biological systems build proteins. In the next part, we’ll see how our DNAs are able to get specific amino acids to make proteins that we need to function well.
III. Nitrogenous bases
Before we get into the nitty-gritty of things, let’s first get into how our body decides stuff, such as our hair color. We know that organisms have DNA and that this DNA has all of the information about how we look, who we are, and all that fancy stuff. DNA has this information that we should have brown hair; RNA, a single-stranded nucleic acid, will take this genetic information. This information gets passed to the site where amino acid chains are built. Our cells will select the amino acid responsible for giving us that hair color. These amino acids will continue to form a chain or a polypeptide chain that will ultimately build protein.
Why should we care? Well, DNA and RNA are made of nucleotides. These nucleotides are made of sugar, a phosphate group, and a nitrogenous base. See where I’m getting?
Nitrogenous bases are molecules that have nitrogen-containing ring structures (they are called bases since nitrogen can be a proton acceptor). These ring structures comprise the nitrogen bases that carry the codes our RNA uses to make specific amino acid chains.
There are five nitrogen bases, namely: adenine, cytosine, guanine, thymine and uracil. These nitrogen bases can be further categorized into purines and pyrimidines.
A. Purines
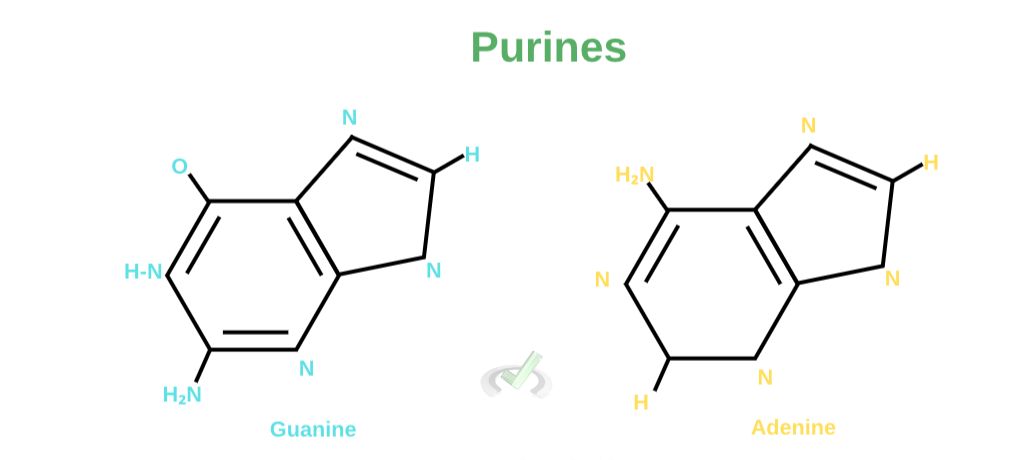
Purines are aromatic compounds that have two fused carbon-nitrogen rings. They have a six-carbon ring merged with a five-carbon ring. Adenosine and Guanine are purines.
B. Pyrimidines
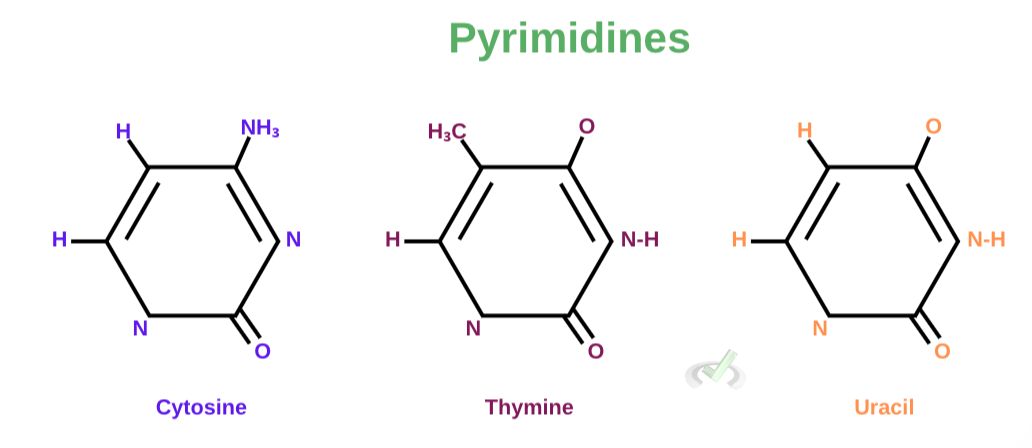
Pyrimidines are aromatic compounds with only one carbon-nitrogen ring. They are much smaller since they only have a ring structure composed of six carbons. Cytosine, Thymine, and Uracil are pyrimidines.
Hydrogen Bonding
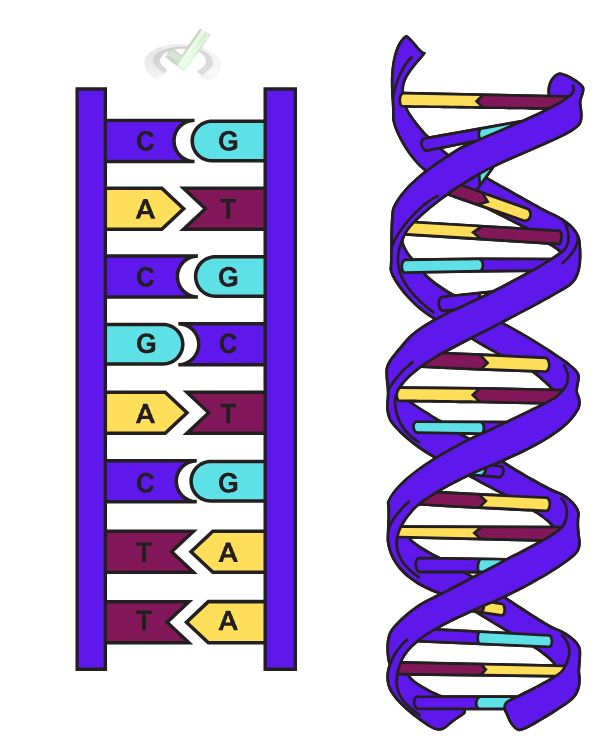
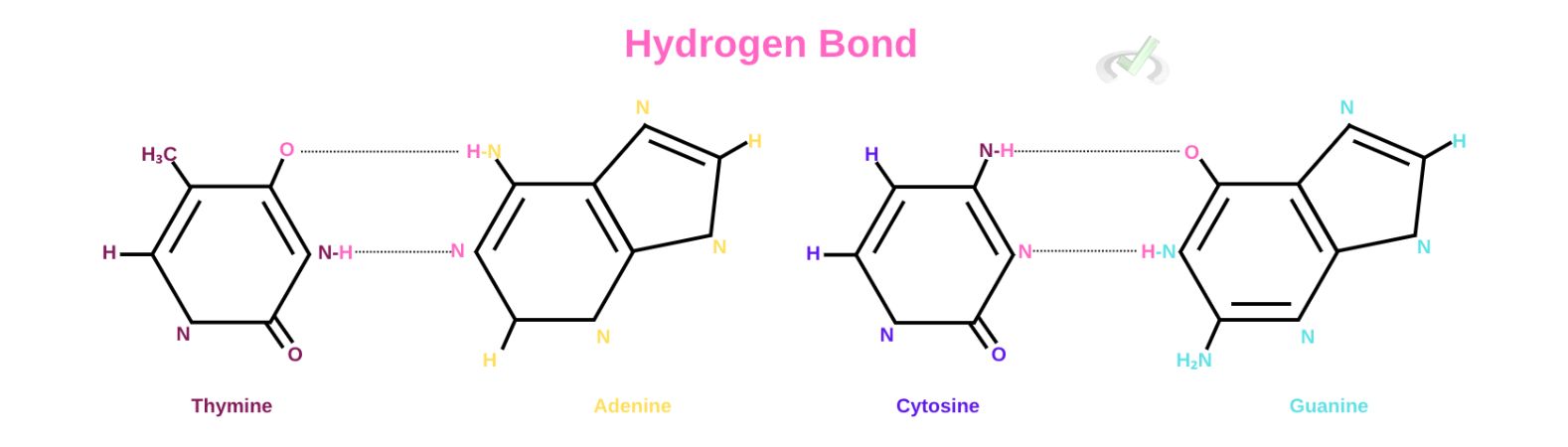
Our DNA carries all the genetic information that makes up everything in our body. It carries information through nitrogenous base pairing. DNA has a double helix structure. This structure holds the genetic codes through these nitrogen base pairs. Purines pair with pyrimidines, which ultimately form this double helix structure from separate strands of RNA. Adenine will pair up with thymine and cytosine will pair with guanine. This pairing serves as a code of genetic information. Uracil only replaces thymine in RNA.
This pairing is made possible through hydrogen bonds between the nitrogen bases.
IV. Conclusion
Amino acids are the building blocks of proteins. An amino acid's structure consists of an amino group, a carboxyl group, and a side chain that characterizes the amino acid. They form peptide bonds to build proteins and it does so through the same nucleophilic substitution reaction. In this process, the amino group of one amino acid attacks the carbonyl atom of a carboxyl group of another. Electrons rearrange until they displace water as a good leaving group. This reaction is also known as condensation since it releases water at the end of the reaction. Other nitrogen-containing compounds include nitrogen bases vital in storing and making genetic information for protein synthesis. These nitrogen bases form a hydrogen bond between other bases during nitrogen base pairing.
V. Key Terms
- Amino acid - the basic unit of a protein with a side chain, a carboxyl group, and an amino group.
- Deoxyribonucleic Acid (DNA) - a double-stranded chain that carries genetic information.
- Ribonucleic Acid (RNA) - a single-stranded molecule that carries and transports genetic information.
- Peptide - A chain of amino acids merged together through peptide bonds.
- Peptide bond - a type of covalent bond that links amino acids together.
- Purines - Nitrogenous bases with two rings of carbon-nitrogen bonds.
- Pyrimidines - Nitrogenous base with one ring of carbon-nitrogen bonds.
VI. Nitrogenous bases
Sample Practice Question 1
How will the genetic sequence ATGATCTCGTAA in DNA be copied onto mRNA?
A. UAGAUCUCGUAA
B. TACTAGAGCATT
C. AUGAUCUCGUAA
D. UAGUAGAGUAUU
Ans. C
mRNA is transcribed from the DNA template by following complementary base pairing:
A (adenine) pairs with U (uracil)
T (thymine) pairs with A (adenine)
C (cytosine) pairs with G (guanine)
G (guanine) pairs with C (cytosine)
The correct transcription results in AUGAUCUCGUAA.
Sample Practice Question 2
Which of the following is NOT TRUE?
A. The amino group acts as a nucleophile in peptide bond formation.
B. There are four nitrogen bases: adenine, cytosine, guanine, and uracil.
C. Peptide bond formation occurs through nucleophilic substitution reaction.
D. Nitrogenous bases pair through hydrogen bonds.
Ans. C
Peptide bond formation occurs through a condensation reaction, not nucleophilic substitution. In this process, the amino group of one amino acid acts as a nucleophile and attacks the carboxyl group of another, releasing a molecule of water.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
