Yes we know… unfortunately we had to tackle this section in one way or another despite some of the not-so-good memories we may have had in organic chemistry I and II. However, just like our journey to medical school, we all start somewhere.
However, this is one of the sections and chapter overviews in organic chemistry that has significant overlap with many other sections and subtopics tested on the MCAT, as what you’ll learn here serves as the basis for many other topics!
This overview will give you the basics of how to identify and name organic chemistry molecules. Let’s dive into it!
Organic Chemistry Nomenclature on the MCAT: What You Need to Know
Topics on organic chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
While this section is important, it’s not so much about the number of questions you’ll be asked; rather, it’ll be your job to identify a molecule via the nomenclature when it comes up on passage content!
Introductory organic chemistry accounts for 15% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems
Important Sub-Topics: Organic Chemistry Nomenclature
As mentioned before, there is some overlap between this chapter overview and other MCAT topics. This is especially true for biochemistry topics as some of the basics covered in this chapter overview will appear in topics like carbohydrate structure, amino acids and proteins, etc.
1. Naming Fundamentals of Organic Molecules
Before getting into the rules of naming organic molecules, let’s give a basic definition of what these molecules are before naming them: organic molecules are simply molecules based on a carbon backbone. The most basic organic molecules are alkanes composed of a straight chain of hydrogen and carbon atoms covalently linked by single bonds.
In addition, instead of being drawn out with each atom and bond, organic chemists often use the “stick” format to represent organic molecules where each point represents a carbon atom while the bonded hydrogen atoms are implied, as shown below!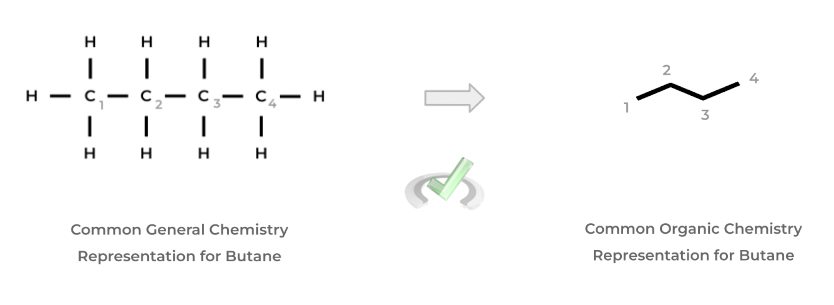
Knowing the names of common alkanes will be key not just for when the MCAT tests on them, but also when more complex structures get built from alkanes such as alkenes, alkynes, and when adding more substituents.
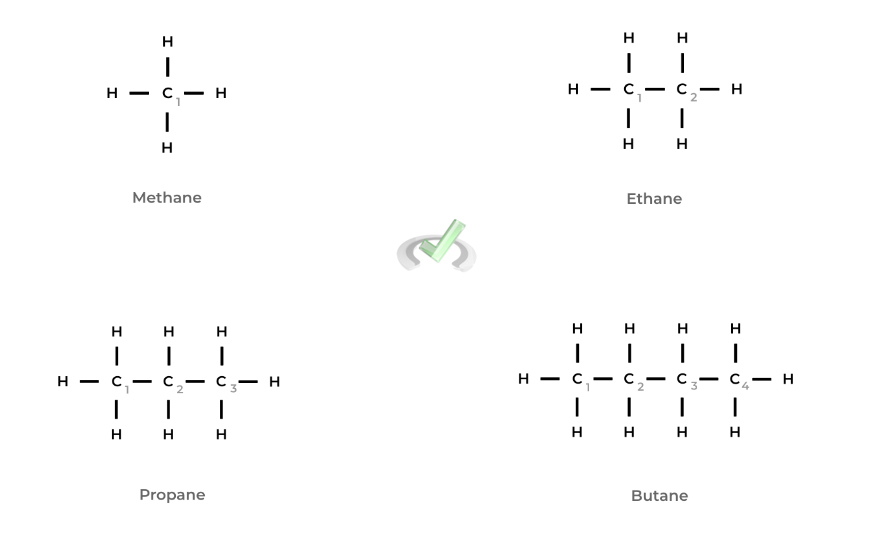
Luckily, the first 4 alkanes are the only ones with unique names, while the rest follow common sense. The first and simplest alkane is methane which only contains 1 carbon bonded to 4 hydrogens. It’s then followed by ethane, propane, and butane.
The following alkanes are named by the common numbering suffixes: a 5 carbon alkane is pentane, a 6 carbon alkane is hexane, a 7 carbon alkane is heptane, and so on.
If a double or triple bond is added to the alkane, the molecule becomes an alkene or alkyne, respectively. Luckily, the prefixes from the alkane names remain the same: the suffix -”ane” simply changes to “-ene” or “-yne” as shown below!
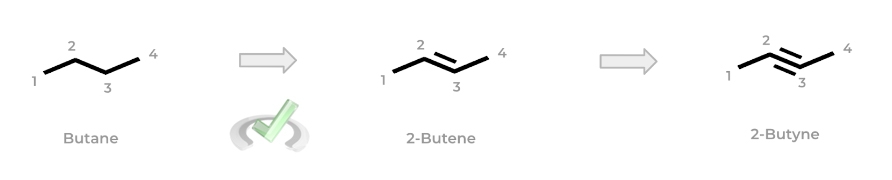
Note also how the number in front indicates the lowest number carbon where the double/triple bond is located! You’ll see this “lowest numbered carbon” motif come up a lot when getting into the specific steps of naming more complex organic chemistry molecules which we’ll introduce in a bit – just keep this in mind for now!
Finally, let’s get into the basic steps of naming these molecules! We can break down naming into 3 main steps: 1) identify the parent chain, 2) identify the substituents/functional groups, and 3) numbering of carbon atoms.
To find the parent chain, we have to find the longest chain of carbon atoms. It’s kinda like drawing a line and seeing how many carbons we can include with just one try. Start off at one free end and try to include as many carbons as possible!
This might take a little getting used to! You’ll have to look at the molecule from different angles and will engage in a lot of trial and error. However, if you remain patient and diligent, you’ll without a doubt see improvement in no time!

Next, from the longest carbon chain, identify any major substituents. We’ll learn about many different substituents and you’ll see that in some cases some substituents will have “priority” in their naming.
This means that the alkane suffix of “-ane” can be changed depending on the priority of the substituents. For example, if a 5 carbon chain contains a carboxylic acid, the suffix changes to “-oic acid” resulting in pentanoic acid instead of pentane. We’ll cover this more later on but just wanted to introduce the idea now.
The most common substituents you’ll encounter are alkyl function groups which are built of alkane chains. These all have prefixes as their alkane counterparts but just have their suffixes to “-ane” and “-yl”.
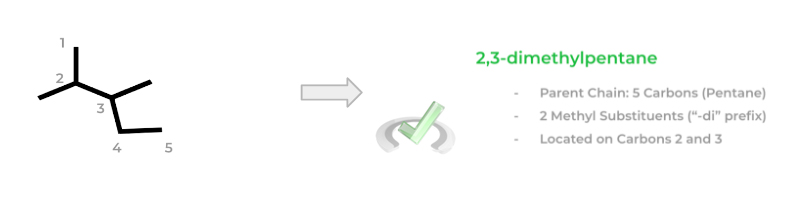
After identifying the various substituents, you can finally number the carbon chain. This might be the trickiest part as you must number the carbons in a way where the first substituent on the chain comes on the lowest possible numbered carbon. This is a little confusing when put in words, so let’s give an example!

Notice how in the left molecule, the methyl group comes on carbon 3 when numbered this way. The right molecule offers an alternative numbering where now the methyl group is bonded on carbon 2 – this is the correct numbering as the first substituent comes on a lower numbered carbon!
You can now start to name the molecule! When naming the molecule, you must take into account the parent chain, the types of substituents (their carbon number and how much of each is present), and the alphabetical order!
If 2 or 3 of the same substituent are present, the prefixes “-di” or “-tri” are added before. Additionally, a number(s) in front of the substituent name is placed in order to indicate their carbon number location.
Finally, the listing of the substituents in the molecule name must follow alphabetical order. For example, if there is a methyl and ethyl group attached to a butane parent chain at carbon 1 and 3 respectively, it’d be named 3-ethyl, 1-methylbutane. Note that the prefixes “-di” and “-tri” are excluded from the alphabetical order!
Full Study Notes : Naming Fundamentals of Organic Chemistry Molecules
For more in-depth content review on naming fundamentals of organic chemistry molecules, check out these detailed lesson notes created by top MCAT scorers.
2. Important Common Names and Substituents
While there are numerous organic chemistry molecules and substituents, you’ll most likely encounter only the most common!! In this section, we’ll introduce a couple more of these common molecules in addition to the ones we covered above!
There are more unique alkyl functional groups that are built from the straight alkyl chains! When you look at them, you’ll notice that they have the same atom type and quantity, but they’re just arranged in a different way!
These different atomic arrangements of the same molecule are actually called isomers, a topic that we cover in our other chapter overview! Take a look at some of the more unique alkyl substituents below!
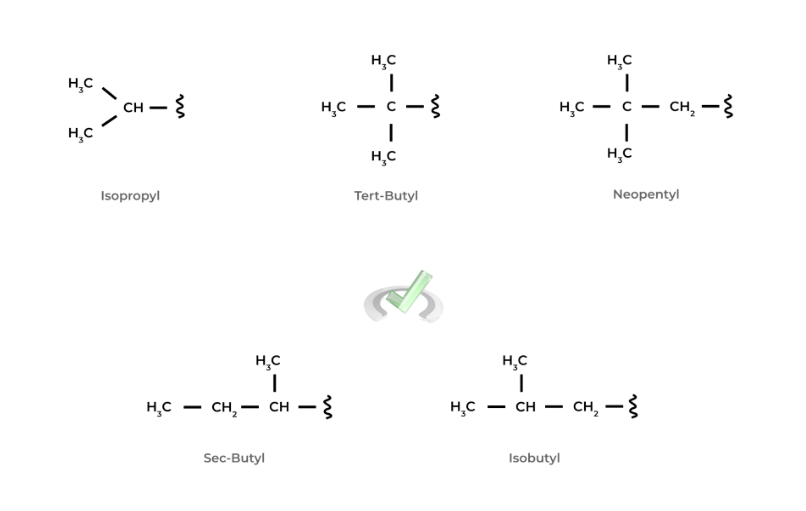
We wanted to include these unique alkyl functional groups because it’s important to note that their names do follow the alphabetical order rule when naming an organic molecule! As you can see, there are lots of little details and nuisances to keep in mind!
Additionally, we’ll introduce 3 main functional groups that you’ll without a doubt encounter on the MCAT: alcohols, aldehydes, and carboxylic acids. Alcohols have a hydroxyl group (-OH) singly bonded to free carbon.
Aldehydes and carboxylic acids are pretty similar as they both contain a carbonyl group (a carbon atom double bonded to an oxygen atom) and are both located at the end of the molecule. However, aldehydes have their carbonyl carbon bonded to a hydrogen atom white carboxylic acids are bonded to a hydroxyl group!
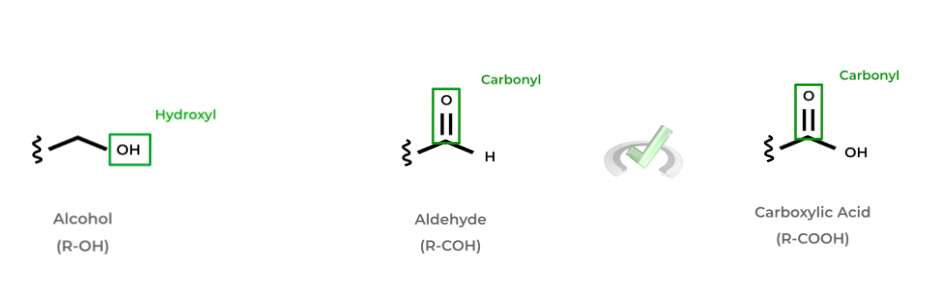
Below are a couple examples of some of the common alcohols, aldehydes, and carboxylic acids. Can you see if you can spot a pattern?
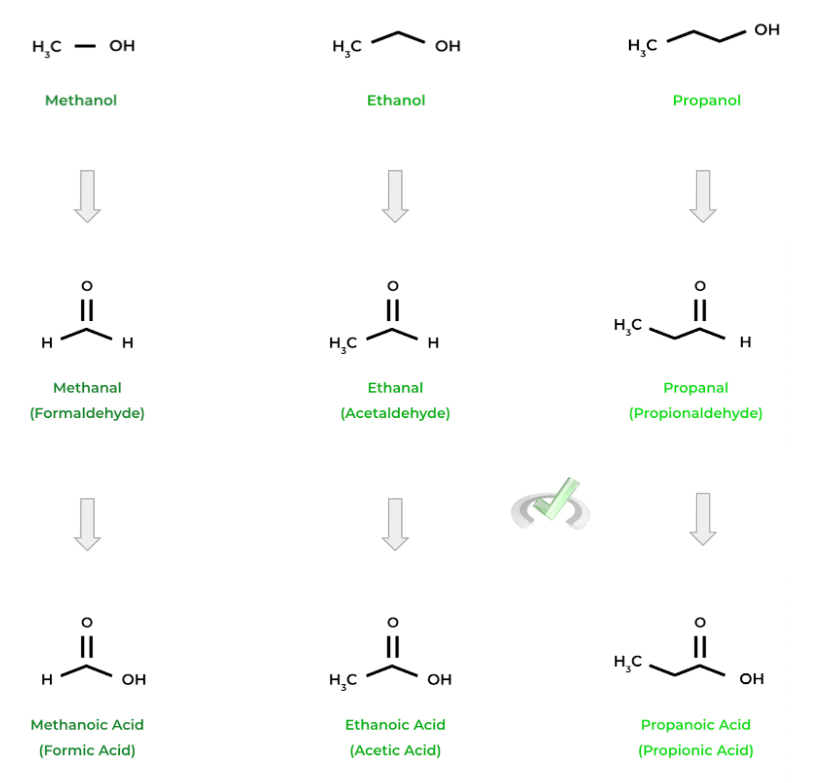
As we can see, the names start with the same prefixes as their alkane counterparts based on the number of carbons (“-meth” for 1 carbon, “-etha” for 2 carbons, etc.)! Note also how the common names for aldehydes and carboxylic acids are similar (i.e. formaldehyde and formic acid)!
Full Study Notes : Most common names and substituents of organic molecules
For more in-depth content review on most common names and substituents of organic molecules, check out these detailed lesson notes created by top MCAT scorers.
3. Priority Ordering of Functional Groups in Organic Chemistry
As we alluded to above, it’s important to know the priority ordering of functional groups in order to correctly name the organic molecule. But why is the priority of the molecule important in the first place?
If we have 2 functional groups, let’s say an alcohol and a carboxylic acid, we have to know which one is “higher” in priority in order to choose the appropriate suffix when naming our molecule.
In this case, the carboxylic acid has higher priority than the alcohol which is why we use the suffix “-oic acid”. Take a look at the example below in order to get a better idea of the concept of functional group priority.
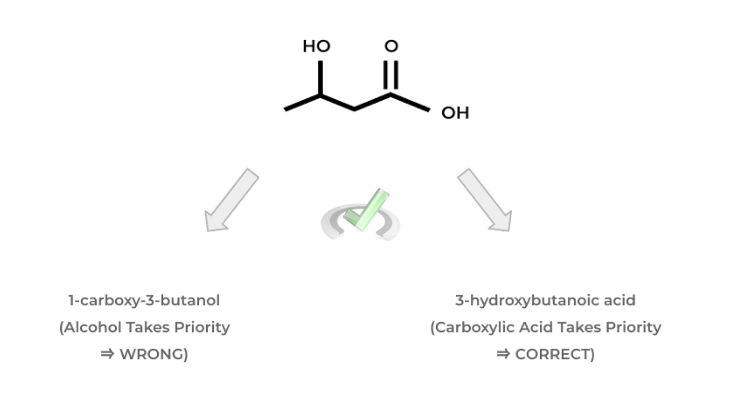
Below is a list of the most common functional groups and their priority! There are a bit more functional groups than the ones shown but the one’s outline is the one you're most likely to encounter!
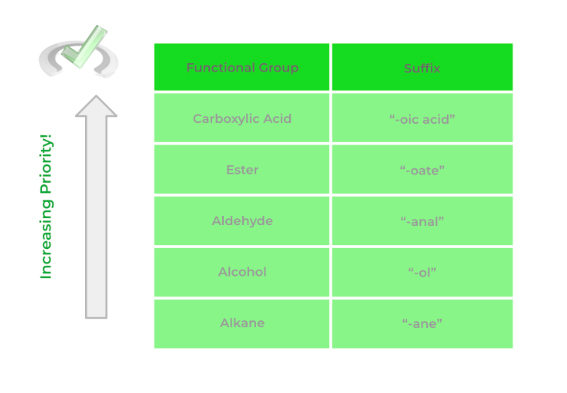
Full Study Notes : Priority Ordering of Functional Groups in Organic Chemistry
For more in-depth content review on priority ordering of functional groups in organic chemistry, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about organic chemistry nomenclature!
Term | Definition |
|---|---|
Parent Chain | Longest chain of carbon atoms in an organic molecule |
Alkane | Simplest organic molecule composed to hydrogen and carbons connected by single bonds |
Alkenes | A derivation of alkanes which contains at least one double bond between 2 carbons |
Alkynes | A derivation of alkanes which contains at least one triple bond between 2 carbons |
Alcohol | Functional group where a hydroxyl group is connected to a free carbon |
Aldehyde | Functional group which contains a carbonyl group and a hydrogen atom bonded to the carbonyl carbon |
Carboxylic Acid | Functional group which contains a carbonyl group and a hydroxyl group bonded to the carbonyl carbon |
Additional FAQs - Organic Chemistry Nomenclature on the MCAT
Is Organic Chemistry Nomenclature on the MCAT?
What is a Prefix in Organic Chemistry Nomenclature? – MCAT
What Are The Steps in Organic Chemistry Nomenclature? – MCAT
What is Additive Nomenclature? – MCAT
Additional Reading Links – Study Notes for Organic Chemistry Nomenclature on the MCAT
Additional Reading -- Organic Chemistry Topics:
- Aldehydes and Ketones on the MCAT
- Bonding on the MCAT
- Carboxylic Acids and Derivatives on the MCAT
- Isomers on the MCAT
- Laboratory Techniques and Separations on the MCAT
- Nitrogen Containing Compounds on the MCAT
- Phosphorus Containing Compounds on the MCAT
- Alcohols and Ethers on the MCAT
- Nucleophiles and Electrophiles on the MCAT
- Spectroscopy on the MCAT
- Redox Reactions Organic Chemistry on the MCAT







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
