Every great architectural building and successfully competing sports team all start with a solid foundation to build upon. In the same way, getting down the fundamentals of nucleophiles and electrophiles will make learning other organic chemical reactions much easier!
If there’s one section that is the “end all, be all,” it’s definitely this one! These subjects and topics covered in this chapter overview and our individual articles provide the basis for almost all the organic chemical reactions that are studied!
After this chapter overview, you’ll be comfortable in understanding the interactions between nucleophiles and electrophiles in organic chemistry.
Nucleophiles and Electrophiles on the MCAT: What You Need to Know
Topics on organic chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
As we emphasized above, this is one of the most high yield sections on the MCAT; however, the way it’ll be tested will be subtle. While you might only have 2-3 questions specifically asking about SN1/2 reactions, you’ll see that you’ll have to establish a foundation from the material learned here in order to answer other questions.
Introductory organic chemistry accounts for 15% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems.
Important Sub-Topics: Nucleophiles and Electrophiles
We want to preface before getting into all of this that for the scope of the MCAT, only a general understanding of these concepts is needed – this isn’t your OCHEM 1/2 midterm or final exam!
Really nitty gritty exceptions, super specific reactions, mechanisms, and definitions are, for the most part, out of the scope of the MCAT. As such, we’ll cover the general principles that’ll suffice for MCAT mastery!
1. Fundamentals of Nucleophiles and Electrophiles
Luckily, these 2 terms basically oppose each other in their definitions. Nucleophiles have the ability to donate an electron pair and are generally electron rich. Electrophiles can accept an electron pair and are generally electron poor.
Does the first part of the definitions sound familiar? That’s because this is a similar definition to Lewis bases and acids! It then comes to no surprise that basicity is a huge factor for determining the nucleophilicity of a species!
A majority of the organic chemistry reactions we’ll discuss essentially deal with the interactions between nucleophiles and electrophiles: an electrophile accepts an electron pair donated by a nucleophile which results in the formation of a bond.
In organic chemistry, most electrophiles involve an electrophilic carbon. This will without a doubt be a common motif to find in many reactions for esters, carboxylic acids, aldehydes, etc.
There are a couple of important factors when assessing how nucleophilic a molecule is. These include basicity, charge, electronegativity, and steric hindrance. A great way to remember the trends is to understand the rationale and logic behind why these trends occur!
Though we don’t want to pick favorites, basicity is probably your first go-to when assessing the nucleophilicity of a species. After all, nucleophiles follow the same definition of Lewis bases. As a species' basicity increases, the nucleophilicity increases.
This is a great trend and generalization because you can look at the relative strength and pKa of the conjugate acid to determine the basicity and thus the nucleophilicity of a species. Look at that figure below!
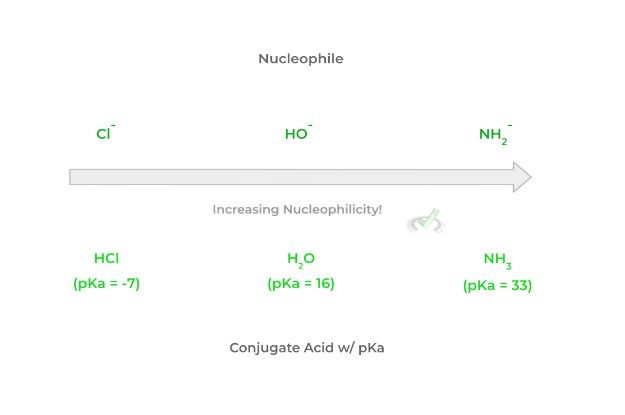
Note that as the strength of the conjugate acid decreases, the basicity of the nucleophile increases which thus increases the nucleophilicity. Remember that the smaller the pKa, the stronger the conjugate acid and vice versa!
In addition to basicity, the charge is also a great indicator of nucleophilicity – an increase in negative charge results in an increase in nucleophilicity. This should make sense as an increased amount of electron density results in an increase in negative charge!
From this, we can make another great rule of thumb that’ll help you on the test: the conjugate base is always a better nucleophile. This is because the loss of the hydrogen atom results in an extra free electron pair adding to the negative charge!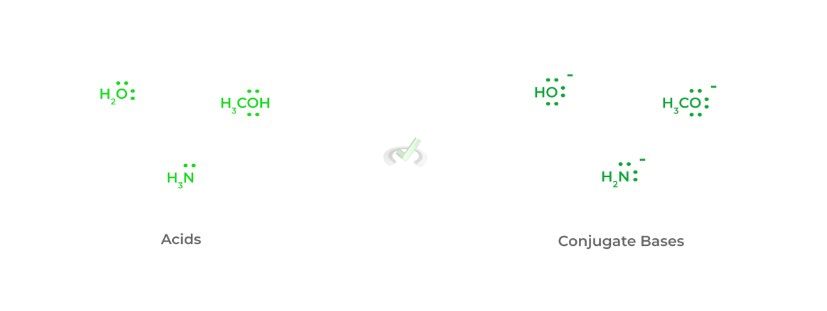
Another factor that helps in determining the nucleophilicity of the species is its electronegativity. In this case, the more electronegative a species is, the less nucleophilic it is.
Recall that the more electronegative a species is, the “tighter” it holds onto its electrons. This tighter hold of electrons will cause a decrease in its ability to donate those electrons!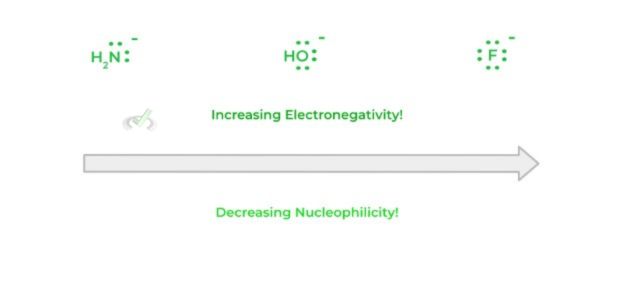
However, it’s important to note that this trend for electronegativity should only be restricted to going across the period on the periodic table. When solvents come into play, we’ll see that the type of solvent can sometimes cause alterations in this trend.
Finally, how bulky a certain species, called steric hindrance, can also affect the nucleophilicity of a species. Namely, the bulkier a species is, the less nucleophilic it is. Nucleophilicity can also be characterized by how fast a reaction can occur; if a molecule is too bulky, this can reduce the reaction rate!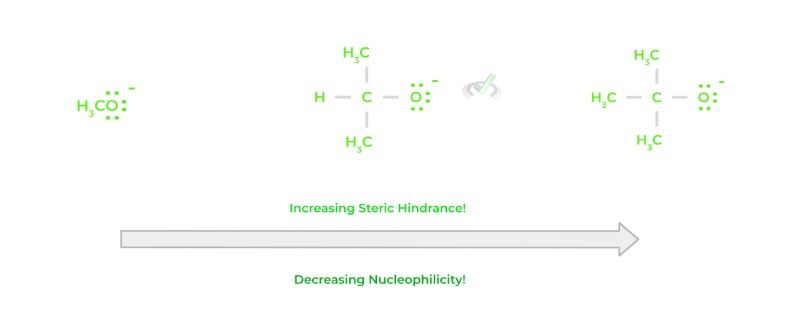
The characteristics of electrophiles are much simpler to explain. Generally, electrophiles have a positive charge or some partial positive charge, such as in the case of a dipole moment!
If there is no positive charge, the electrophile will probably have an atom that doesn’t have a full octet. In other, fancier terms, there’s a free orbital that can accept an electron pair. Look below at a couple of examples!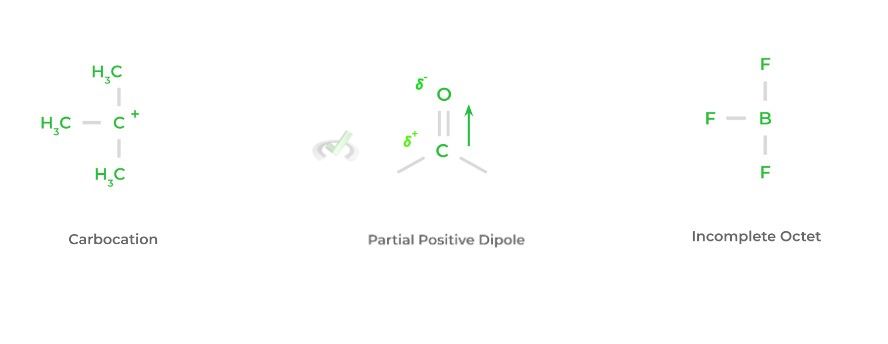
Full Study Notes : Fundamentals of Nucleophiles and Electrophiles on the MCAT
For more in-depth content review on general characteristics of nucleophiles and electrophiles, check out these detailed lesson notes created by top MCAT scorers.
2. The Nucleophilic Substitution Reaction
It’s imperative that come test day, you’re at least familiar with the general overview and characteristics of this reaction as this is a fundamental reaction that serves as the basis for many of the other reactions you’ll cover for the MCAT!
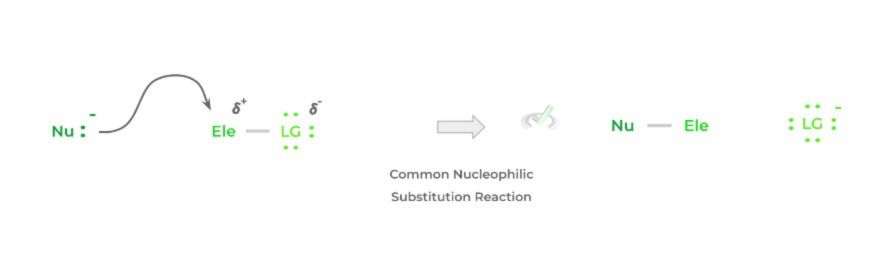
Overall, the reaction is fairly simple and a lot can already be inferred based on its name! In its bare form, a nucleophile will target an electrophilic atom, usually an electrophilic carbon, and donate a pair of its electrons, forming a bond.
In order to compensate for the additional bond formed, a previously attached species to the electrophilic carbon will be released – this species is termed the leaving group. Below is a simplified representation of the reaction.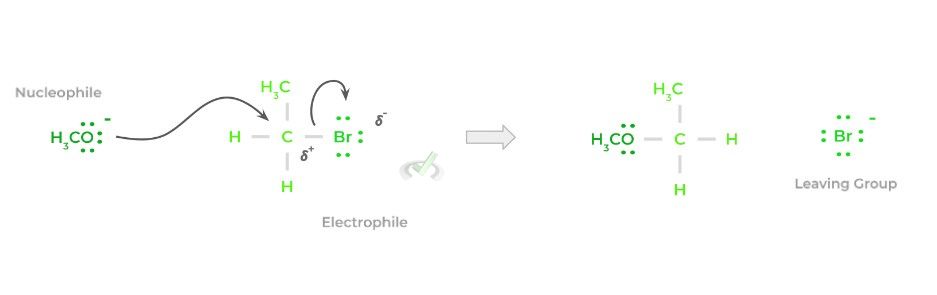
Though the above is a very simple and general example, this is essentially the basis for all nucleophilic substitution reactions! While the main players of the reaction are the nucleophile and electrophile, we also must take into account the leaving group!
The great thing about leaving groups is that they’re essentially the opposite of what a good nucleophile is. While a good nucleophile is a strong base, good leaving groups are generally weak bases!
Because the good nucleophile is a strong base that wants to donate its electron pair, the weak base characteristic of the leaving group allows it to accept an electron pair. We can look at the strength and pKas of a leaving group's conjugate acid to determine its base strength – the stronger the conjugate acid (lower pKa), the weaker the base.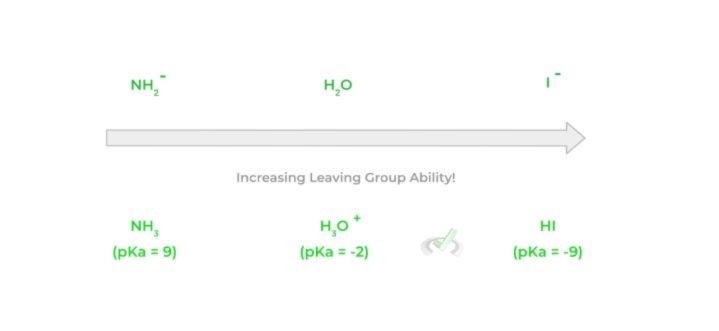
Full Study Notes : The Nucleophilic Substitution Reaction on the MCAT
For more in-depth content review on nucleophilic substitution reactions and leaving group characteristics, check out these detailed lesson notes created by top MCAT scorers.
3. SN2 v.s. SN1 Reactions
When it comes to nucleophilic substitution reactions, there are actually 2 main types: SN2 and SN1. Both involve a nucleophile substituting a leaving group, but mainly differ in the mechanism and the stereochemistry of the products!
In an SN2 reaction, the action of the nucleophile attacking and forming a new bond as well as the release of the leaving group, occur at the same time! In other words, the actions occur all in one step!
An important phrase to associate with SN2 reactions is “backside attack.” This is because when the nucleophile attacks and eventually make a bond with the electrophile, the new product has the opposite stereochemistry to the original reactant (i.e., change from an (R) to an (S) configuration).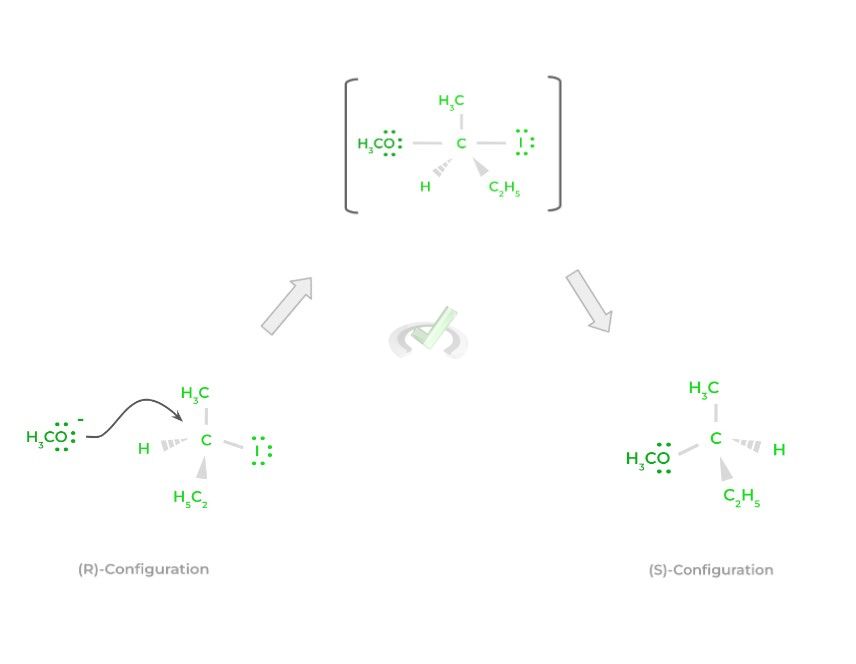
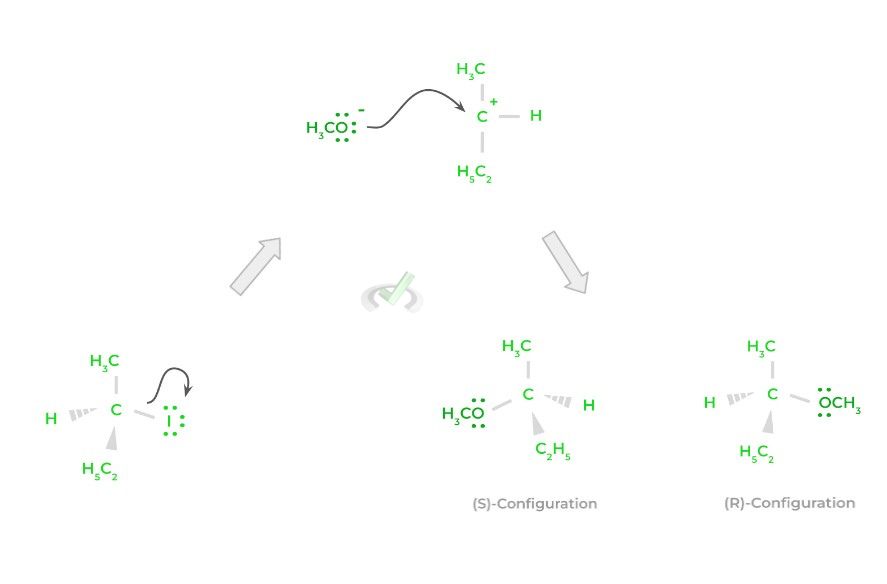
Let’s now contrast this to the SN1 reaction! For an SN1 reaction, there will be 2 steps: in the first step, the leaving group will be released even without the attack from a nucleophile – this results in the formation of a planar carbocation.
In the second step, the nucleophile attacks the positively charged carbocation and donates its electrons to form the new bond. Because the nucleophile attacks a planar carbocation structure, the resulting product will have an equal 50:50 ratio of (R) and (S) configurations, termed racemic.
Contrast this to the SN2 reaction, where the resulting product will always have the opposite stereochemistry as the reactant. Because the carbocation in an SN1 reaction can be attacked from either side, a racemic mixture is produced.
Full Study Notes : SN2 v.s. SN1 Reactions on the MCAT
For more in-depth content review on the differences between SN2 and SN1 nucleophilic substitution reactions, check out these detailed lesson notes created by top MCAT scorers.
4. Effects of Solvents on Nucleophilicity
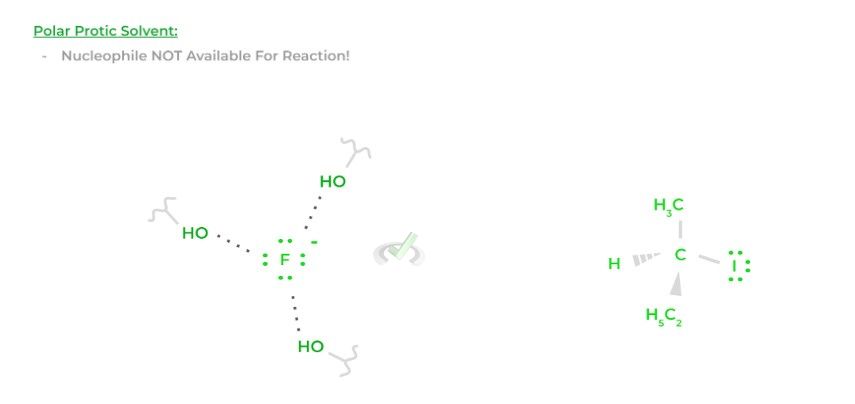
Organic chemical reactions are sometimes performed in solvents, usually polar protic or aprotic solvents. Interestingly enough, the type of solvent can also affect the nucleophilicity of a species. Let’s look at a fluoride ion as an example!
Polar protic solvents are capable of forming hydrogen bonds. If fluoride is acting as a nucleophile in a polar protic solvent, the solvent can begin to form hydrogen bonds with the fluoride, which decreases its availability to participate in the reaction, causing a decrease in nucleophilicity.
It’s kinda like when there’s a really good and skilled basketball player – like Devin Booker – on the court. Because he’s so good, he draws so much attention from the defense that he’ll end up getting double or triple-teamed!
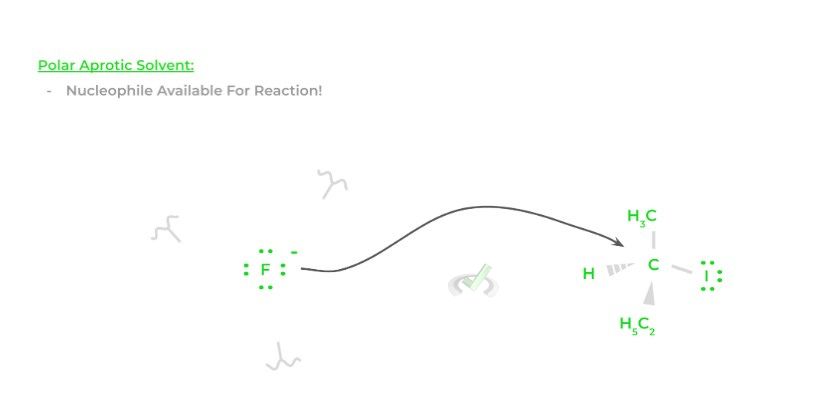
When the reaction occurs in polar aprotic solvents, hydrogen bonds cannot form and can’t disrupt the nucleophile’s availability allowing it to participate in the reaction, which increases the fluoride ion’s nucleophilicity!
Full Study Notes : Effects of Solvents on Nucleophilicity on the MCAT
For more in-depth content review on how solvents affect nucleophilicity, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about nucleophiles and electrophiles in organic chemistry!
Term | Definition |
|---|---|
Nucleophile | An electron rich species which can donate its electron pair to form a bond; Generally very strong bases |
Electrophile | An electron poor species which can accept an electron pair to form a bond: Generally have complete (or partial) positive charge or incomplete octet |
Nucleophilic Substitution Reaction | Organic chemistry reaction where a nucleophile attacks and donates its electron to form a bond with an electrophile; Results in the release of a leaving group previously on the molecule |
Leaving Group | The species that is released during a nucleophilic substitution reaction and is replaced by the nucleophile |
SN1 Reaction | A type of nucleophilic substitution reaction which occurs in 2 steps: 1) the release of a leaving forming a carbocation and 2) nucleophilic attack; Results in a racemic mixture of the products |
SN2 Reaction | Type of nucleophilic substitution reaction which occurs in one step: the nucleophilic attack and release of the leaving group both occur simultaneously; Results in the product always having the opposite stereochemistry than the reactant |
Racemic | A 50:50 mixture of molecules with opposite stereochemical configurations |
Polar Protic Solvent | A type of polar solvent which can form hydrogen bonds and potentially decrease a species nucleophilicity |
Polar Aprotic Solvent | A type of polar solvent which cannot form hydrogen bonds and doesn’t not affect the nucleophilicity of a species |
Additional FAQs - Nucleophiles and Electrophiles on the MCAT
What is the Difference Between Nucleophiles and Electrophiles? – MCAT
Electrophiles, conversely, are electron poor species that have the ability to accept an electron pair to form a bond. Because nucleophiles usually target and donate their electrons to electrophiles, electrophilic species generally have a complete (or partial) positive charge or an incomplete octet.
What Makes a Good Nucleophile? – MCAT
When going across a period, however, increasing electronegativity will decrease nucleophilic strength. Similarly, as a compound is more sterically hindered, its nucleophilicity decreases.
How Do You Remember Nucleophiles and Electrophiles? – MCAT
Do You Need to Know Organic Chemistry Mechanisms? – MCAT
The MCAT is a multiple choice based test, so just having the bare minimum basics will suffice when you’re tested on organic chemical reactions. Instead, try and focus on what the resulting product of a reaction would be given the reactants and conditions!
Additional Reading -- Organic Chemistry Topics:
- Aldehydes and Ketones on the MCAT
- Bonding on the MCAT
- Carboxylic Acids and Derivatives on the MCAT
- Isomers on the MCAT
- Laboratory Techniques and Separations on the MCAT
- Nitrogen Containing Compounds on the MCAT
- Phosphorus Containing Compounds on the MCAT
- Organic Chemistry Nomenclature on the MCAT
- Alcohols and Ethers on the MCAT
- Spectroscopy on the MCAT
- Redox Reactions Organic Chemistry on the MCAT







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
