We've previously discussed the electron cloud model. In this model, we described the atom as a particle with a nucleus at the center and a cloud surrounding it.
The nucleus contains both the proton, a positively charged particle, and the neutron, a neutral particle. Surrounding the nucleus is a cloudy space where the negatively charged electrons move around.
I. Energy Levels
We must look at Bohr's model to understand how electrons move around. In his model, we see orbits surrounding the nucleus, similar to how the planets orbit around the sun. These orbits are called energy levels. The closer an electron is to the nucleus, that is, at the orbit closest to the nucleus, the more stable it is.
In this model, the electron is the most stable at the 1st energy level. This stability is due to the attraction between the electron's negative charge and the proton's positive charge. The farther the electron is from the nucleus, the lesser the electrostatic force between the electron and the proton. The electron constantly moves around this orbit as a response to the attractive force from the nucleus.
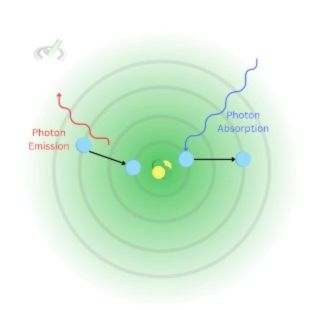
II. Atomic Absorption and Emission
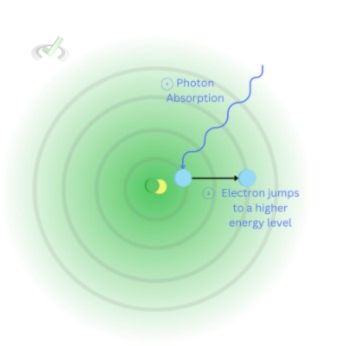
Electrons can absorb energy. A photon is a particle that can be absorbed or released by electrons. When an electron absorbs a photon, it absorbs energy. When this energy is strong enough, it allows the electron to move into a higher energy level.
Think of it this way, when an electron is stable, it doesn't have enough energy to fight its attraction with the proton. But when a photon arrives, it gets strong enough to break away from that attraction and move into a much higher energy level.
Looking at the image, we see the electron absorbing photon at the first energy level. It jumps to a much higher energy level when it gets enough energy.
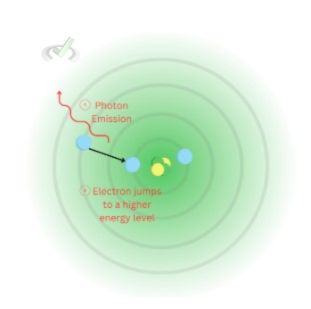
Being at a higher energy level also means that it's not at its most stable state. When an electron is at an energy level wherein it's not as stable, it tends to move back to a more stable energy level.
Photon emission can also occur from other causes, such as thermal and electrical energy. However, the idea is the same. When an electron returns to a lower energy level, it releases the photon/energy it has absorbed.
III. Calculations
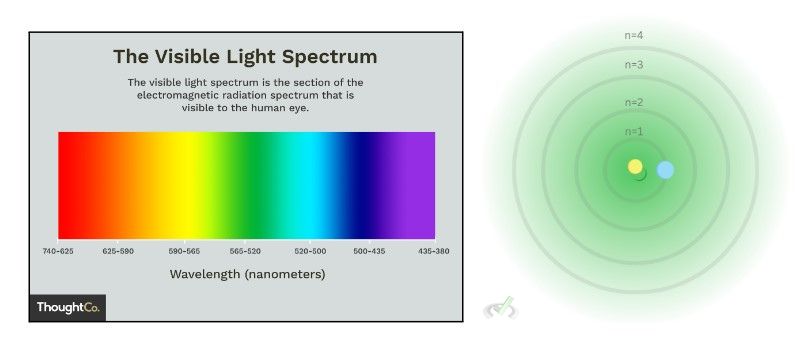
The colors visible to us have a specific wavelength. We can see colors because these are the colors within the visible spectrum or our eyes can see. These colors can reflect light with wavelengths particular to that color. The trees look green because they reflect the wavelength within the "green wavelength range", or 520-565 nanometers.
The electron falls back into a much more stable energy level during emission. It releases a photon as a result of the previous excitation. We can calculate the wavelength it releases and determine what color this emission shows us.
We can calculate an electron's change of energy using the Rydberg constant for hydrogen, Rₕ₁ which is equal to 2.17910⁻¹⁸ Joules
To get the change of energy of an electron during emission, we use the following formula:
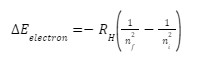
Where ni/f represents the energy level in the initial and final states, respectively.
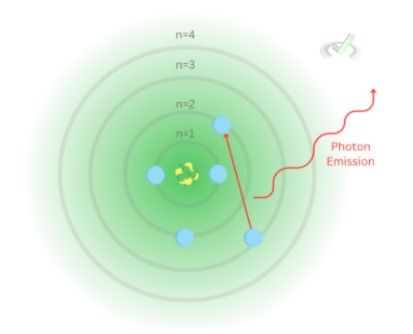
Using the image below where the electron jumps from the third to the second energy level, we get:
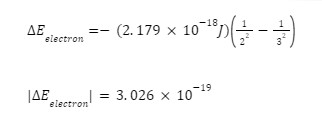
From our previous article, we’ve also established the equation for the wavelength which is:
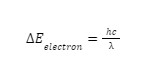
Where E = Energy
h= Planck’s constant
c = Speed of light
λ = Wavelength
Using this equation, we get:

In the emission spectrum, the wavelength we got corresponds to red visible light.
IV. Conclusion
The electron cloud model represents how the atom looks like. Electrons move around the nucleus in orbits. When an orbit is close to the nucleus, the attraction between the negatively-charged electron and the positively-charged nucleus is strong, making it the most stable orbit or energy level.
The farther an electron is from the nucleus, the weaker the attraction between the two. When an electron gets excited in the form of a photon or an outside energy source, it gets excited enough that it can jump to a different energy level. This process of absorbing energy from a photon is called Atomic Absorption.
The electron gains enough energy to repel the attractive force it has with the nucleus. At the higher energy level, the forces are weak, thereby making the electron unstable. The energy that it absorbs from the photon will always have to be released back. When the electron goes back to a more stable energy level, it releases a photon. This photon reflects light as a result of the release of energy coming from the electron.
The light we see can reflect different colors that correspond to various wavelengths along the visible spectrum. As such, when a photon is released, it reflects light, and we can use various formulas to calculate the wavelength or the energy released as a result of atomic emission.
V. Key Terms
- Atomic Absorption - absorption of energy, usually through photons, that results in electrons jumping to a higher energy level.
- Atomic Emission - release of energy followed by electrons moving to a more stable energy level.
- Energy Level - a cloudy path similar to an orbit where electrons can move around. The first energy level is the most stable energy level.
- Wavelength - The length of the 'peaks' of light waves that correspond to a particular color.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
