Aside from sounding like a word you wanna throw around when you wanna sound like Einstein, thermodynamics is another important concept and topic that’ll show up and be tested on the MCAT!
Just by the name itself, you can already get a sense of what’s being studied in thermodynamics: “-thermo” refers to thermal energy which we’ll define in a bit and the “-dynamic” portion refers to the flow or movement.
After reading this chapter overview, you’ll be able to understand the basic underlinings of thermodynamics! Let’s dive in!
Thermodynamics on the MCAT: What You Need to Know
Topics on thermodynamics will be tested on the Chem/Phys section of the MCAT and can appear both as passage based and fundamental discrete questions.
Thermodynamics is one of the more high yield physics and chemistry topics tested so try and expect around 6-9 questions that can probably appear!
Introductory physics accounts for 25% of the content covered in the Chemical and Physical Foundations of Biological Systems.
Important Sub-Topics: Thermodynamics
Similar to some of the physics chapter overview, you’ll see a lot of overlap with this chapter overview and our “Thermochemistry on the MCAT!” overview! However, this overview goes more into the theory behind thermodynamics while the latter will be centered more on calculations.
In addition, it may be best to go over our “Work and Energy on the MCAT!” overview as a couple of definitions and concepts covered there reappear here as well!
1. The Role of Heat in Thermodynamics
Let’s first distinguish between what heat and thermal energy is as sometimes these terms are used interchangeably which is somewhat incorrect. Recall that energy is simply a term to describe the capacity of a system to do work. Remember the phrase: you need energy to do work.
Thermal energy refers to the energy stored as a result of the vibration of the particles within the system. In other words, it’s simply the kinetic energy in a microscopic scale, referring to the particles.
Heat (often designated as q) refers to the transfer of thermal energy between 2 objects or systems! Another way to think about heat is that it’s a form of energy in transfer.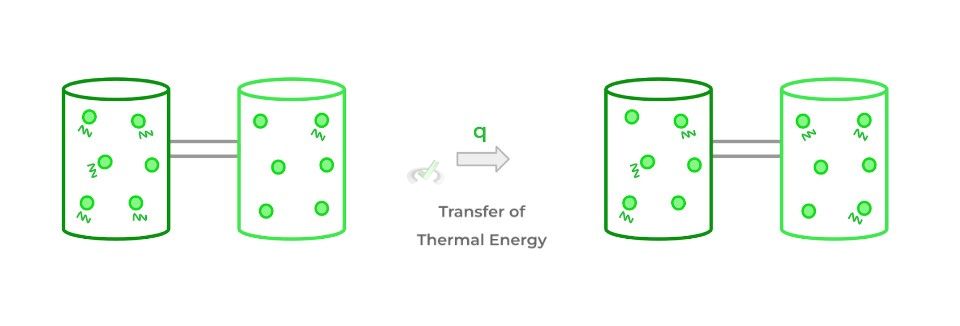
As shown above, the zigzag lines represent the vibrations and kinetic energy of the particles. When thermal energy is transferred in the form of heat, it reaches an equilibrium as shown above!
The dark green system originally has a higher amount of energy compared to the light green system. However, after thermal energy is transferred between the thermodynamic systems in the form of heat, they reach an equilibrium where now the vibration and kinetic energies of the particles are equal.
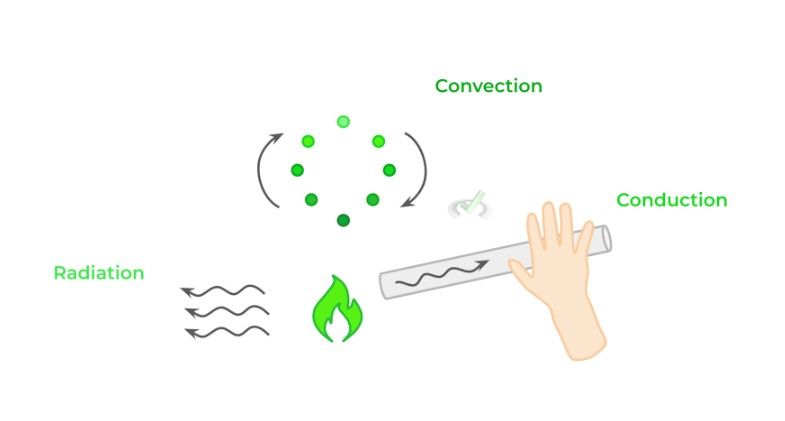
There are 3 main methods of heat transfer: conduction, convection and radiation. Conduction needs direct contact between 2 objects for heat transfer to occur. Convection involves heat transfer via the flow of fluids, namely liquids and gasses. Finally, radiation refers to energy transfer via the release of electromagnetic waves.
Full Study Notes : The Role of Heat in Thermodynamics
For more in-depth content review on the role of heat in thermodynamics, check out these detailed lesson notes created by top MCAT scorers.
2. The Laws of Thermodynamics
Just like other physics topics, there are various laws that govern thermodynamics. There are 4 basic laws: the zeroth, first, second, and third law of thermodynamics, each of which can be modeled by an equation.
The zeroth law of thermodynamics is actually relatively intuitive, but the actual wording might be a little confusing. In words, the law states that if 2 bodies (A and B) are in thermal equilibrium with another body (C), then A and B are in equilibrium. However, the main idea to understand is that when 2 objects are in thermal equilibrium, no net heat flow occurs!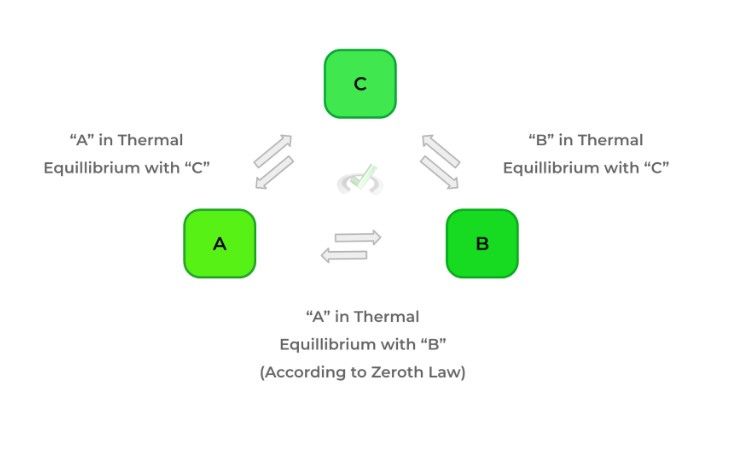
The first law of thermodynamics can be summed up by the phrase “energy cannot be created or destroyed.” In other words, it ensures the conservation of energy when there is a transfer of energy within a system.
As stated by most textbooks, the first law states that the change of internal energy within a system is equal to the amount of heat that flows into or out of the system subtracted by the amount of work done by or on the system.
Note that the internal energy of the system can increase or decrease and depends on the signs of Q (heat) and W (work).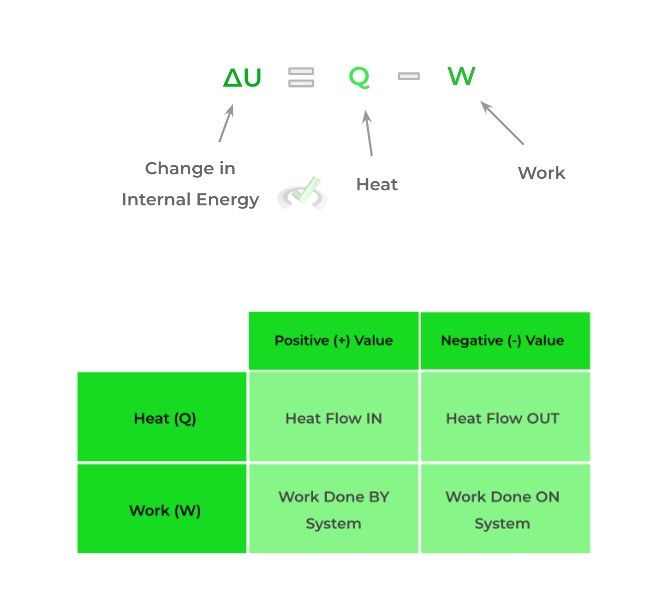
The second law of thermodynamics is often taught as the entropy (S) of the universe is always increasing, also rephrased as the disorder of the universe is always increasing. Another iteration of the second law states heat flows from higher temperature objects to lower temperature objects in order to achieve thermal equilibrium.
A better way to think about the 2nd law of thermodynamics is that energy will be dispersed when permitted to do so. Consider gas particles trapped in a closed container; when a hole is punctured in the container, the gas particle disperses out spontaneously!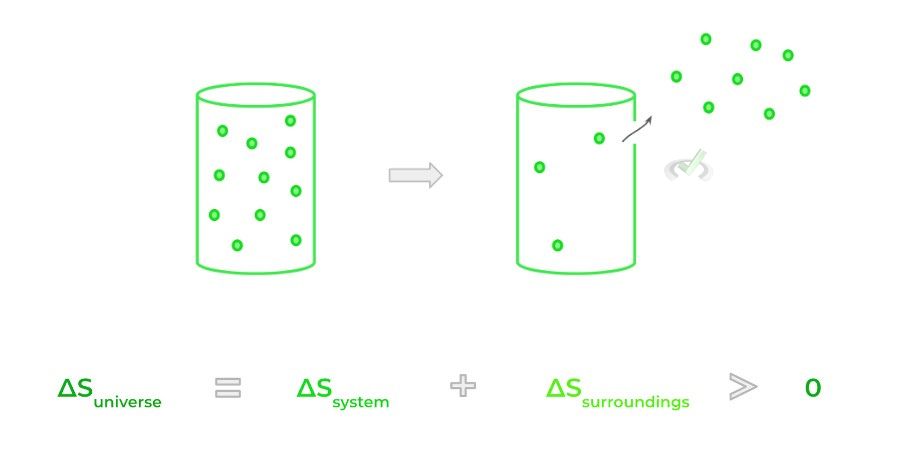
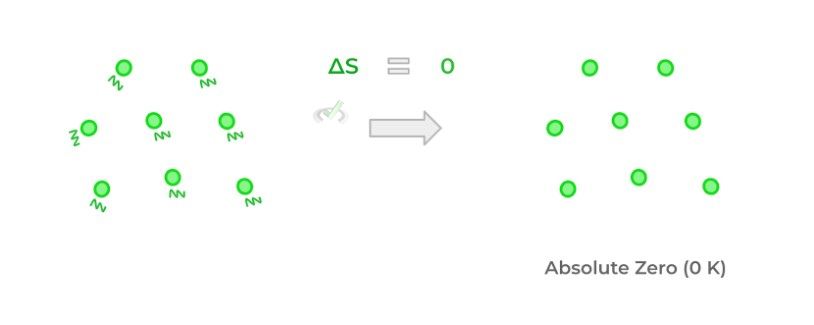
Finally, the third law of thermodynamics basically states that at a theoretical temperature called absolute zero, there is no thermal energy and thus no kinetic energy within the particles. As a result, entropy is also zero because no movement results in no disorder.
Full Study Notes : The Laws of Thermodynamics
For more in-depth content review on the laws of thermodynamics, check out these detailed lesson notes created by top MCAT scorers.
3. Thermodynamic Systems, Functions, and Processes
Let’s now define some key terms in order to get a better overall picture of how to study thermodynamics. We’ve tossed around the word system a lot in our discussion, which basically refers to the portion of the universe with enclosed boundaries that we’re studying, like a container. The surroundings simply refer to the rest of the universe, not including the system.
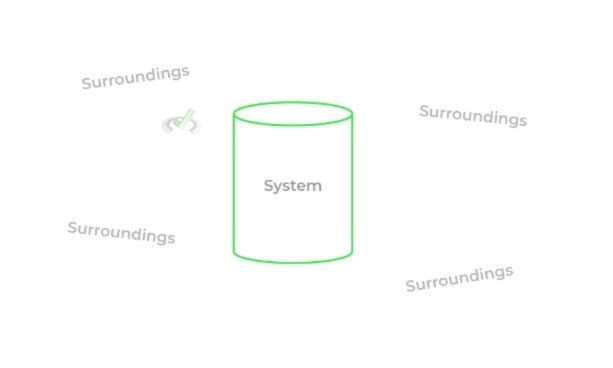
There are different types of systems that basically differ on whether or not they can exchange matter and/or energy with the surroundings!
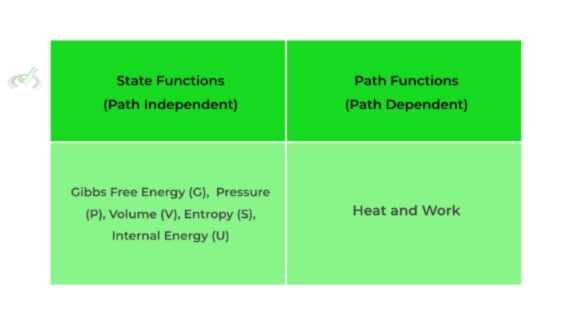
Furthermore, thermodynamic functions are basically thermodynamic properties that can describe the current state of equilibrium of a system. These functions can be divided into either path or state functions. Path functions do depend on the path it takes to get to the equilibrium state while state functions do not.
Some examples of state and path functions are given below. Right now, don’t focus too much on the actual theoretical explanation; rather, just take and internalize these facts. We’ll delve into a more complete explanation in our article.
Finally, we can describe the equilibrium changes in a system via thermodynamic processes. Sometimes when there is a change in equilibrium, we can hold a certain value constant such as volume, temperature, etc.!
When we hold these values constant, we can simplify the equation found in the first law of thermodynamics. As such, each process has its own specific characteristics. Look at the figure below!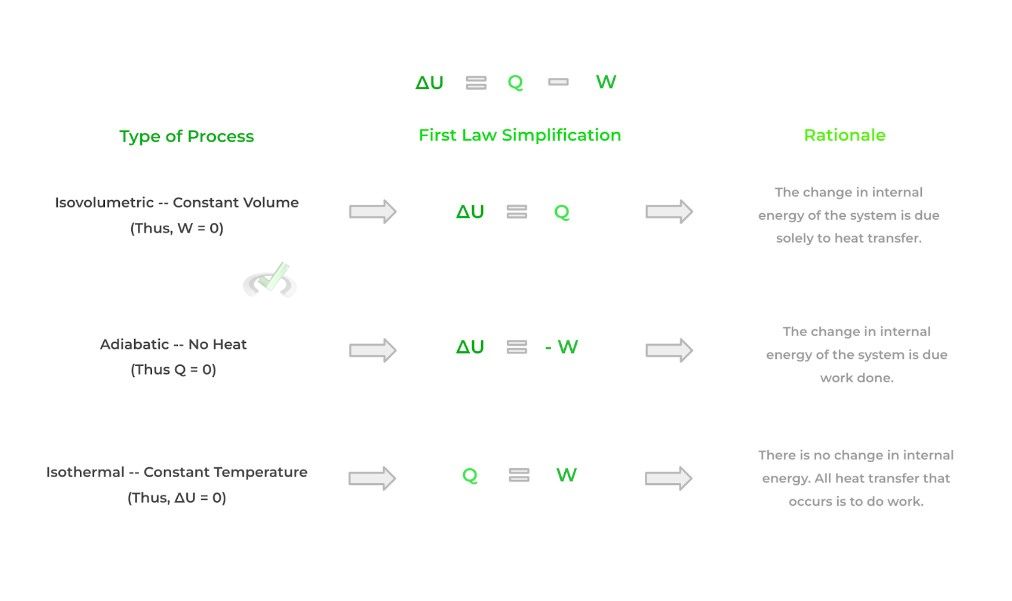
It’s no lie that thermodynamics is one of the more conceptually complex topics on the MCAT, but as long as you stick to the main ideas, you’ll be golden!
Full Study Notes : Thermodynamic Systems, Functions, and Processes
For more in-depth content review on thermodynamic systems, functions, and processes, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about atomic and nuclear phenomena!
Term | Definition |
|---|---|
Heat | The process of energy transfer; can also be thought of as energy in transfer |
Thermal Energy | Refers to the total amount of thermal energy within a system |
Conduction | Form of heat transfer which requires direct contact between 2 objects |
Convection | Form of heat transfer which requires the flow of fluids, namely liquids and gases |
Radiation | Form of heat transfer which requires the emittance of electromagnetic waves |
Entropy | Generally described as the disorder of particles |
System | Enclosed portion of the universe that is being thermodynamically studied |
Surroundings | The rest of the universe outside of the system |
State Functions | Thermodynamic equilibrium properties which IS NOT dependent on the path taken to get to that equilibrium |
Path Functions | Thermodynamic equilibrium properties which IS dependent on the path taken to get to that equilibrium |
Additional Reading Links – Study Notes for Thermodynamics on the MCAT
Additional Reading: Physics Topics on the MCAT:
- Circuits on the MCAT
- Electrostatics on the MCAT
- Fluids on the MCAT
- Kinematics on the MCAT
- Light and Optics on the MCAT
- Magnetism on the MCAT
- Atomic and Nuclear Phenomena on the MCAT
- Units and Dimensional Analysis on the MCAT
- Waves and Sound on the MCAT
- Work and Energy on the MCAT







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
