I. Thermodynamic Systems
As you learn more about thermodynamics, you’ve probably noticed how often certain terms, like systems and surroundings, get mentioned. This is not a coincidence because studying thermodynamics involves these two things!
System
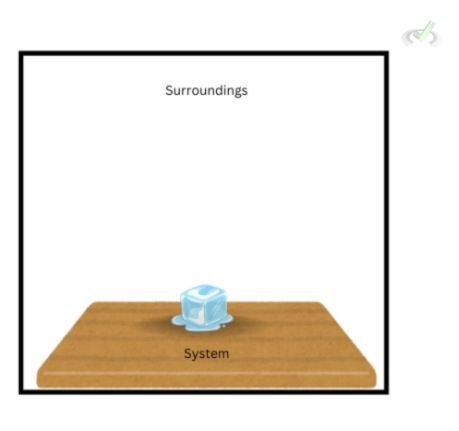
Our introductory article defined the system as the part of the universe we’re studying. It’s the object that we’re interested in and the thing we observe. In the example of a block of ice sitting on top of a table, the block of ice on the table is our system. It’s the thing we want to see where change takes place. A system helps us establish boundaries regarding what we want to measure and observe. By setting the block of ice on top of a table as the system, we isolate the object of interest from everything surrounding it.
Surroundings
If we define the system as the object we’re interested in, the surroundings are anything that is not the system. By the name itself, the surroundings refer to everything that surrounds the system, including the universe. While it can be easily assumed that the surroundings are not as important as the system, they can actually show how the system affects it or how energy can move from the system to the surroundings.
Types of Systems
Observing systems is crucial to understanding how energy can or cannot flow in and out of them. Systems are defined by the type of exchanges between them and their surroundings. When we observe systems, we usually talk about three types: open systems, Closed systems, and Isolated systems.
A. Open System
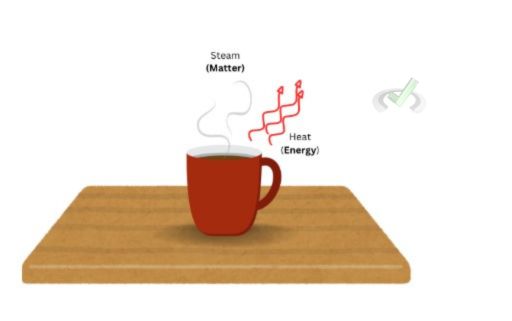
An open system allows the exchange of energy and matter between the system and its surroundings. A cup of hot coffee is an open system because it allows the exchange of energy in the form of heat from the coffee to its surroundings. As a cup of coffee releases steam, it also releases matter in water.
B. Closed System
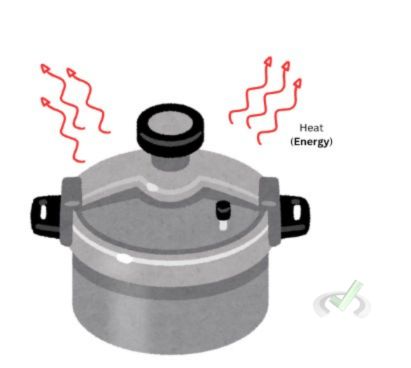
A closed system only allows the exchange of energy with its surroundings. Unlike an open system, closed systems do not allow matter to escape or enter. When we cook using a pressure cooker, matter does not escape from the system; only heat or energy leaves the pressure cooker.
C. Isolated System
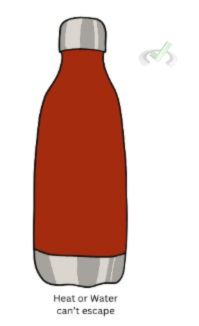
An isolated system does not allow the exchange of energy and matter between it and its surroundings. It completely isolates energy and matter from its surroundings, and no heat or matter escapes the system. An insulated flask is a good example of an isolated system. The water inside a flask can be kept cold or hot since the flask does not allow energy or matter to leave.
II. Thermodynamic Functions
Thermodynamic functions are properties that describe a system's current state of equilibrium. They can be categorized as path or state functions.
A. Path Functions
By the name itself, path functions depend on the path one takes to reach a particular state. These properties define the process–not the state at the end. Examples of path functions are work and heat.
Consider a piston and we compress the gas from one volume to another. If we compress it at a constant temperature, the work will change depending on the volume ratio. If we compress the gas without any heat, the work will depend on the pressure, volume, and heat capacity.
Another way we can think of it is by how we calculate heat and work. We do not have a constant way to calculate these values as we first need to determine what properties are kept constant and what changes are needed.
B. State Functions
State functions are much simpler to understand. These are properties that only depend on initial and final states. In our previous article, we mentioned the change in internal energy a few times, which the difference in heat and work can calculate. Internal energy is a state function calculated using its initial and final state. Entropy, Enthalpy, Pressure, Volume, and Gibbs Free energy are other examples of state functions.
III. Thermodynamic Processes
We can keep one value constant when there is a change in equilibrium. When we hold certain values constant, we can use the first law of thermodynamics to explain certain thermodynamic processes.
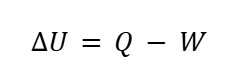
A. Isovolumetric (Constant Volume) Process
When volume is held constant, we can assume that no work is done (W=0). The first law of thermodynamics becomes:
This indicates that the changes in the internal energy in the system come from heat transfer alone.
B. Adiabatic (No Heat) Process
When Q=0, the first law of thermodynamics becomes
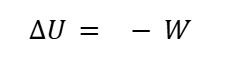
This means that changes in the system's internal energy are the result of its work.
C. Isothermal (Constant Temperature) Process
At constant temperature, there is no change in the system's internal energy U=0. The first law then becomes:
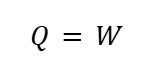
This indicates that when internal energy and temperature do not change, heat transfer occurs solely to do work.
IV. Conclusion
In thermodynamics, setting a system is important to see the bounds of the area we want to observe. Open functions allow the exchange of matter and energy, closed functions only allow the exchange of energy, and isolated systems do not allow any exchange of matter or energy between the system and its surroundings. Thermodynamic functions can be categorized according to how they’re measured. Path functions depend on their path or the properties that change from one state to another, whereas state functions only rely on their initial and final states. Thermodynamic processes define the changes in energy, heat, and work depending on certain properties that can be kept constant. These concepts sound complex, but they are crucial in defining how properties affect each other.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
