I. What are Amino Acid Reactions?
If you’re coming from the “Amino Acid – Structures” Study Article, welcome back and glad to have you here! Luckily, this topic is a little more easier to understand and contains much less information and content to digest and memorize.
While we usually associate amino acids with structure, names, letter abbreviations, characteristics, etc., it’s also important to know the certain reactions that amino acids are involved in. These not just provide the backbone for protein formation, but can also be a great tool to review some ochem concepts!
This article might be a little easier and lighter, but more importantly, take the time to build bridges and make connections between what’s covered in the article to other topics while studying for the MCAT. Here, really focus on making connections and bridges with other topics on the MCAT!
II. Main Reactions of Amino Acids
There are 2 main types of reactions that amino acids are involved in: 1) Condensation (Dehydration Synthesis) and 2) Hydrolysis. Let’s delve into them a bit more and see why they’re so important to amino acids!
A. Condensation (Dehydration Synthesis)
As indicated by its second name, this reaction involves the synthesis of a bigger molecule by joining 2 smaller molecules together. In the process, it releases a water molecule, hence the term “dehydration”.
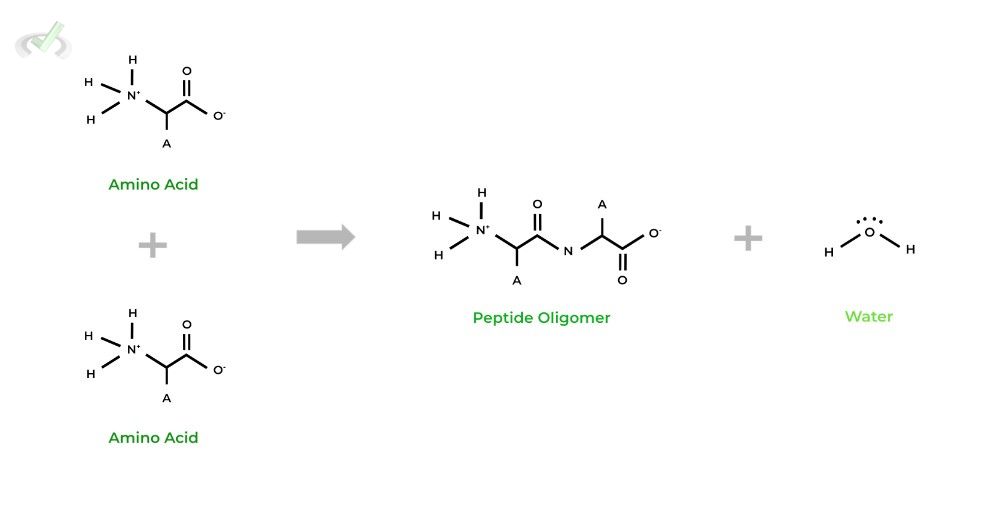
The reaction results in the formation of a peptide bond, which is made between the carboxyl group of one amino acid and the amino group of another amino acid.
Condensation reactions are the basis for protein synthesis, which can be thought of as multiple condensation reactions taking place in order to build polypeptide chains!B. Hydrolysis
Similarly, as implied by its name, this reaction involves the breaking of a bigger molecule into smaller molecules. This is best thought of as essentially the opposite of a condensation reaction!
Notice how in this reaction, instead of it being produced, a water molecule is actually consumed in order to catalyze the lysis of the peptide oligomer!
The inclusion of the water molecule is important when actually considering the organic reaction mechanism, as we’ll touch on soon!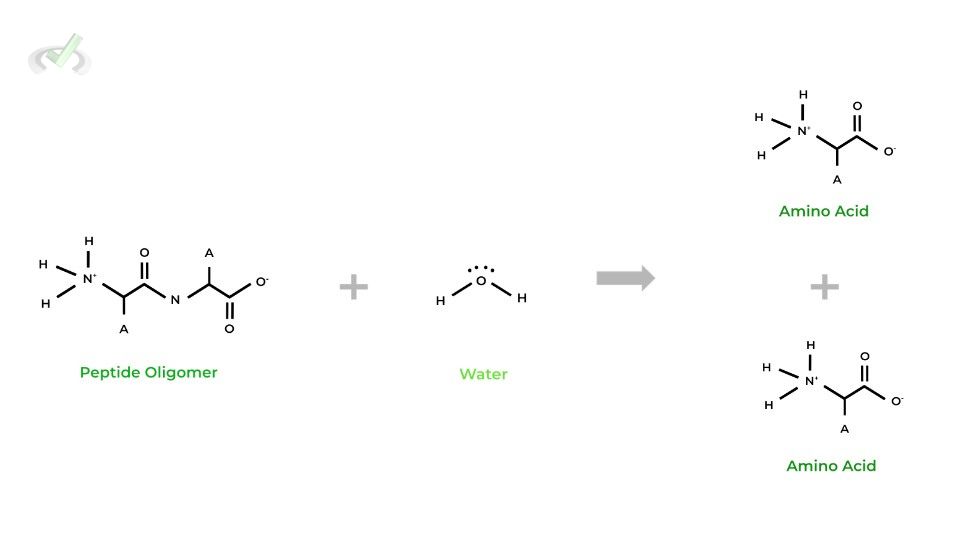
III. Bridge/Overlap
Unfortunately, we have to call back to the (still haunting) fundamentals of organic chemistry, even when talking about amino acids and their associated reactions!
I. Nucleophilic Substitution Mechanism for Peptide Bond Formation
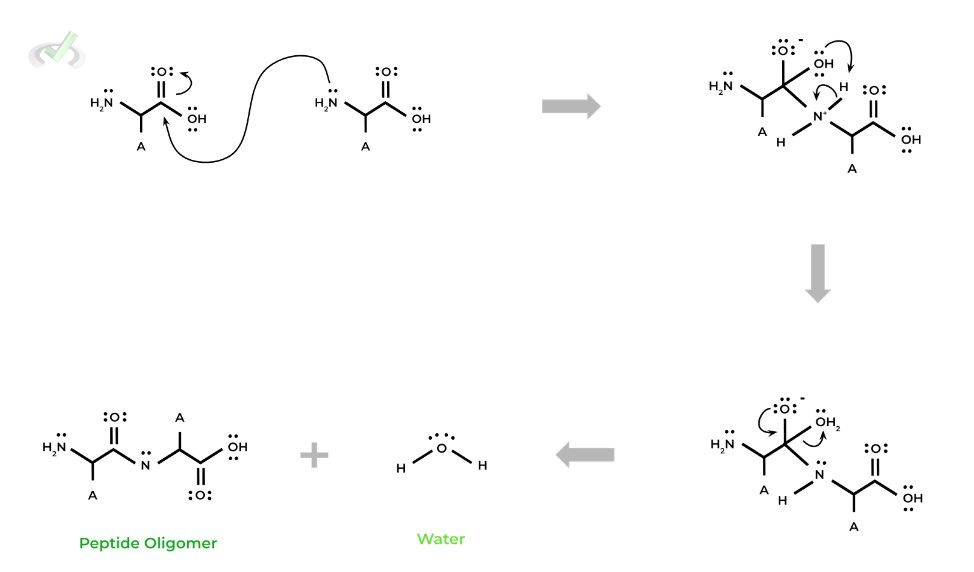
One way that peptide bond formation can be explained, on top of it being a condensation reaction, is to model it also as an organic nucleophilic substitution.
In peptide bond formation, the amino group acts as the NUCLEOPHILE, as it has a set of lone pairs which can nucleophilically attack the carbon on the carboxyl group. which acts as the ELECTROPHILE. Take a look at the mechanism below!It’s not that important to memorize the mechanism for this reaction (sorry about the flashback about your organic chemistry classes & exams!), but more to recognize this reaction is a nucleophilic substitution & to recognize this overlap!
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Condensation (Dehydration Synthesis)
Amino acids can undergo a condensation reaction which results in the formation of a bigger, peptide molecule! As noted by the name, a resulting water molecule also emerges as a product of the reaction in addition to the peptide oligomer.
This ultimately results in the formation of a peptide bond, made between the carboxyl group of one amino acid and an amino group of another amino acid.
These reactions are the basis of protein synthesis as it can be thought of as multiple condensation reactions taking place in order to build polypeptides.B. Hydrolysis
As implied by its name, this reaction results in the breakdown of the larger, peptide molecule into the smaller amino acids, essentially undoing the action of the condensation reaction!
Notice here that instead of being produced, a water molecule is actually consumed in order to catalyze the reaction, which is relevant for the mechanism as it acts as the nucleophile!V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
Which of the following proteins would you expect to utilize hydrolysis as part of its functioning?
A. Steroid Transport Protein
B. Proteases
C. Passive Transport Ion Channels
D. Viral Capsid Proteins
Ans. B.
Proteases are a special class of proteolytic enzymes which work to degrade proteins by breaking the peptide bonds that form the primary polypeptide chain. As such, proteases utilize hydrolysis reactions in order to break these polypeptide bonds back into amino acids.
Steroid transport proteins & passive transport ion channels should not be expected to use hydrolysis because the functions of these proteins are strictly for transport purposes.
Likewise, viral capsid proteins should not be expected to use hydrolysis because it functions strictly for structural purposes.
Sample Practice Question 2
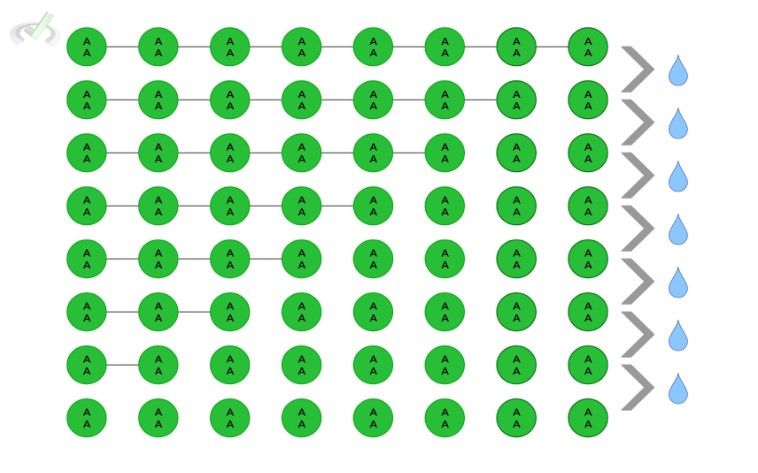
Given a polypeptide composed of 8 amino acids, how many water molecules are needed in order to break the polypeptide chain back to the 8 individual amino acids?
A. 5
B. 6
C. 7
D. 8
Ans. C.
This is actually a trick question so make sure you count the number of water molecules correctly! It is important to note that in the last round of hydrolysis, the remaining polypeptide will split into 2 amino acids! It might be better if there is a visual, as the one shown below







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot