I. What is the ATP Synthase?
Congratulations! You’ve grinded and covered all the other study articles leading to this one! You’ve made it to the last step involving oxidative phosphorylation where we generate the much needed ATP that the cell needs to power all the necessary cellular processes.
While covering this article, you can think of this as an extension of the article covering the electron transport chain, as ATP synthase is sometimes referred to as complex V, just like the other complexes embedded in the inner mitochondrial membrane.
Another great thing about reviewing and studying ATP synthase is that there are quite a few crossovers in terms of content we’ve studied before! Keep that in mind while you’re going through the article, especially in the bridge section!
II. ATP Synthase
Let’s run it back one more time and get y’all situated with a brief overview of the process before getting into the structure and function of ATP synthase, as well as some key terms!
A. Overview of ATP Synthase
ATP synthase is the final step before generating ATP in oxidative phosphorylation, where the enzyme powers ATP synthesis via the flow of H+ ions from the intermembrane space down their electrochemical gradient.
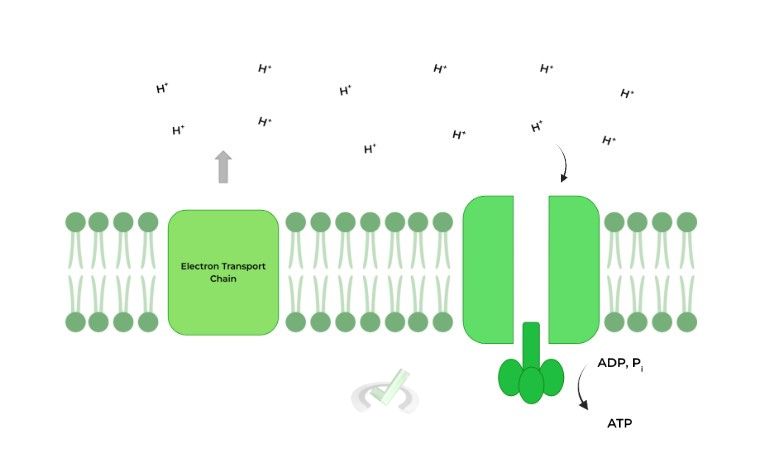
B. Chemiosmotic Coupling and the Importance of the Electrochemical Gradient
We’re so sorry again that we have to bring up this phrase, but we just have to reiterate the importance of it! You can simply think of oxidative phosphorylation as coupling an exergonic reaction to an endergonic one.
Here, the downhill flow of H+ ions represents is exergonic while ATP synthesis is an endergonic one. This makes sense because energy needs to be inputted in order to generate ATP!C. ATP Synthase
We’ll cover ATP synthase by dividing the content into covering the structure of ATP synthase and then its function.
I. Structure
Though there are many nuances and complexities to the structure of ATP synthase, for the scope of the MCAT, we’ll keep it fairly simple by covering only the F0 and F1.
The F0 component allows for the passage of protons down their electrochemical gradient and the F1 component catalyzes the production of ATP.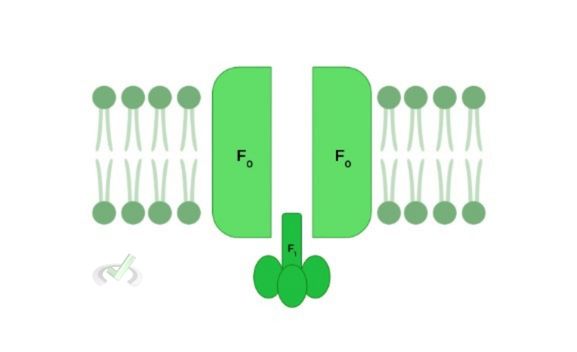
II. Function
As mentioned before, the flow of protons DOWN their electrochemical gradient via the F0 component powers the catalyzation of ATP synthesis via the F1 portion.
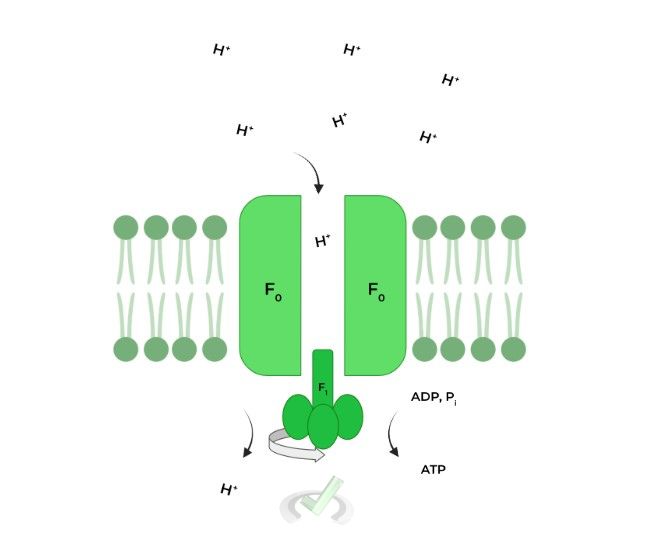
A widely accepted model to explain ATP synthesis by the enzyme is rotary catalysis. The F1 component has 3 subunits which all have different conformations: 1) ADP, Pi, 2) ATP, and 3) empty, set up like a circle!
Specifically, the downhill flow of 4 ions powers 1 rotation: this rotation causes the ADP, Pi conformation to switch to the ATP conformation where the ADP, Pi is synthesized to ATP.
In addition, this also causes the previous ATP conformation to switch to the empty conformation, where the ATP is then released!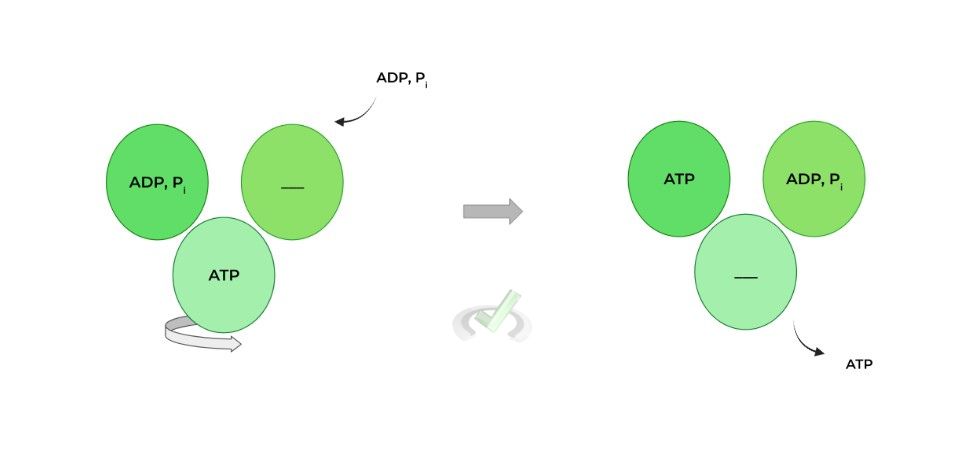
D. Calculations
As mentioned above, an important stat to note and memorize is that 4 H+ ions are needed to power the rotation. This explains why one molecule of NADH yields 2.5 ATP while FADH2 only yields 1.5 ATP.
In one round of the ETC, NADH can pump 10 protons because it’s oxidized by complex I. Here, complex I, III, and IV pump out 4, 4, and 2 H+ ions respectively, yielding 10 total H+ ions pumped.
However, because FADH2 is oxidized later in the ETC by complex II, only 6 total H+ ions are pumped by 1 FADH2 via complex III and IV which pump 4 and 2 H+ ions, respectively.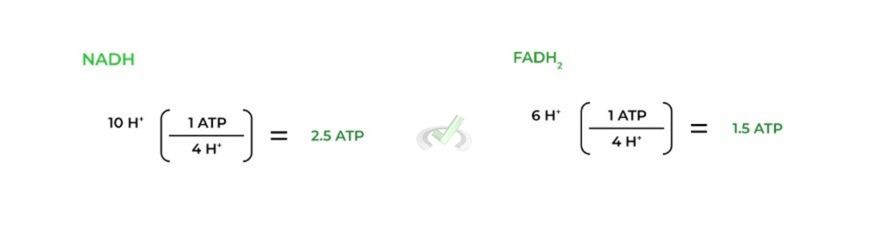
Because 4 protons power the production of 1 ATP, we can do a simple division to find out the ATP yield per NADH and FADH2 molecule, as shown above!
III. Bridge/Overlap
Did the mechanism for ATP synthesis by ATP synthase look familiar? That’s because the mechanism has some similarities to secondary (2˚) active transport! Let’s see how they compare to each other!
I. Secondary (2˚) Active Transport
Recall that secondary active transport occurs when the DOWNHILL flow of an ion/particle powers the UPHILL flow of another ion/particle!
This is because the downhill ion/particle flow is EXERGONIC, as it’ll release energy during the process. This released energy powers the ENDERGONIC uphill flow of the other ion/particle!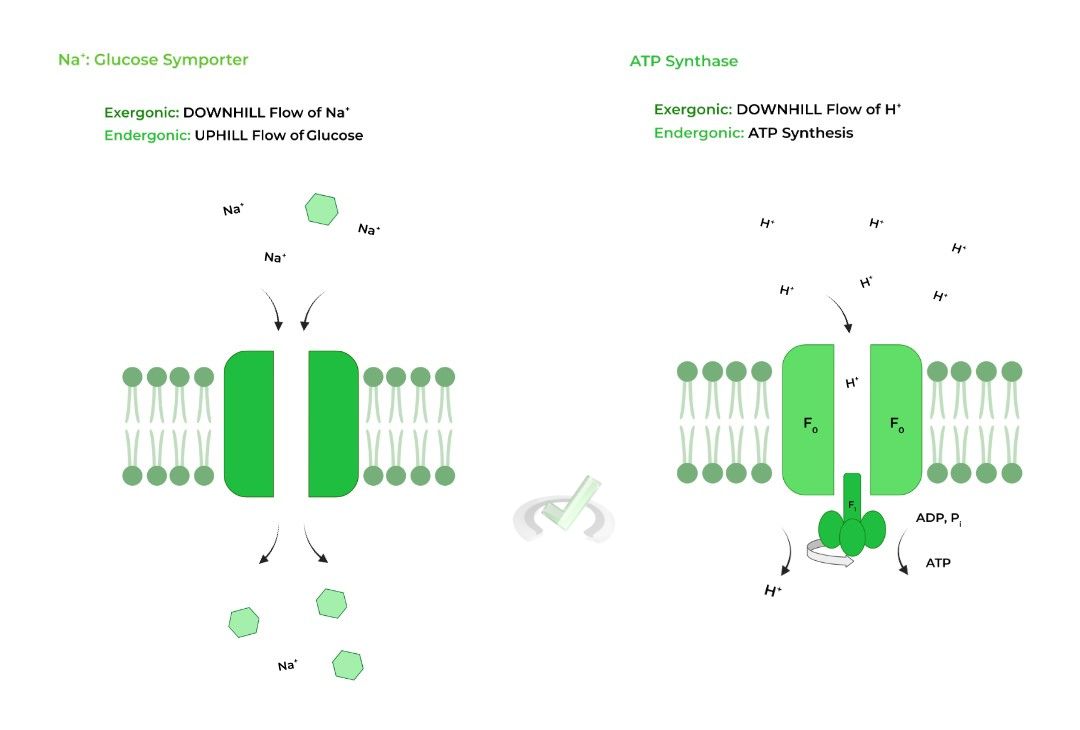
Hopefully the similarity is a little more clear! In this case, ATP synthase also allows for the release of free energy via the exergonic downhill flow of protons. However, instead of powering the uphill flow of a substance, the free energy powers the endergonic production of ATP!
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Overview of ATP Synthase
ATP Synthase catalyzes the final portion of oxidative phosphorylation, by utilizing the flow of H+ ions down their electrochemical gradient to power ATP synthesis.
B. Chemiosmotic Coupling and the Importance of the Electrochemical Gradient
ATP synthase essentially couples an exergonic reaction to power an endergonic one: the exergonic downhill flow of H+ ions releases energy.
This released free energy is then utilized to power the endergonic processes of ATP synthesis, similar to 2˚ active transport!
C. ATP Synthase: Structure and Function
We’ll cover ATP synthase by dividing and covering it via its 1) structural components and 2) how it specifically functions!
I. Structure
The 2 main structural components of ATP synthase for the scope of the MCAT are the F0 and F1 components.
The F0 portion allows for the flow of H+ ions back into the mitochondrial matrix while the F1 portion is the main site for ATP synthesis.II. Function
The F1 portion actually has 3 binding sites, all with different conformations: 1) ADP, Pi, 2) ATP, and 3) empty.
The flow of H+ ions powers the rotation of these binding sites which changes their conformation. The ADP, Pi changes to an ATP conformation which catalyzes the synthesis of ADP and Pi to ATP.
The ATP changes to an empty conformation where the ATP is released to be utilized by the cell!D. Calculations
As mentioned, 4 protons need to flow down the F0 component to power 1 rotation of the F1 portion and thus 1 ATP!
This results in every molecule of NADH producing about 2.5 ATP while one molecule of FADH2 produces about 1.5 ATP.
This is due to the order of the ETC: because NADH is oxidized first at complex I, a total of 10 protons can be pumped (10H+/4+ = 2.5 ATP). However, because FADH2 is oxidized later at complex II, it can only pump 6 protons (6 H+/4+ = 1.5 ATP)IV. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1:
2,4-dinitrophenol is a compound known to be an uncoupler, meaning it allows for the protons in the intermembrane space to leak back into the matrix. How would this affect ATP synthesis?
A. ATP synthesis would decrease
B. ATP synthesis would increase
C. ATP synthesis would remain the same
D. ATP synthesis would increase, then decrease
Ans. A
ATP synthase is powered via the downhill flow of H+ ions to allow for the catalyzation of ATP synthesis. However, if the protons are already dissipating and moving back into the matrix, there will be minimal to no protons that will flow down fia the F0 portion of ATP synthase.
As such, ATP synthesis will decrease because of the decreased H+ ion flow through ATP synthase.
Sample Practice Question 2:
Efrapeptin is a fungal peptide that binds and inhibits the function of the F1 component of ATP synthase, while still leaving the F0 component properly functioning. Given this, how should ATP production be affected and how does the acidity of the matrix change?
A. ATP synthesis increases; matrix acidity increases
B. ATP synthesis increases; matrix acidity decreases
C. ATP synthesis decreases, matrix acidity increases
D. ATP synthesis decreases, matrix acidity decreases
Ans. C
Because the F1 portion is inhibited, ATP synthesis will decrease. However, because the F0 component is still intact and functioning, the protons can still utilize this component to flow back into the mitochondrial matrix.
As H+ ions flow back into the matrix, the acidity should increase!







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
