I. What is Enzyme Reversible Inhibition?
Welcome back! (assuming that you’re coming from our enzyme kinetics study article); now we can focus on the real life application of understanding why it’s important to be comfortable with the basics of enzyme kinetics.
Enzyme inhibitors are exactly what they sound like, referring to how various molecules (often drugs!) work to inhibit the function of enzymes by affecting their Km and vmax values. While there are other methods of enzyme inhibition, such as feedback inhibition or irreversible inhibition, we’ll mainly be focusing on reversible enzyme inhibition.
Understanding enzyme inhibition is important as this is another high yield topic that the MCAT tests for. This is due to it’s importance in medicine as well as in biological function. Additionally, enzyme inhibition is applicable in MCAT questions regarding your ability to interpret graphs and data via the Lineweaver-Burk plots..
These are all topics which we will definitely go over in the article!
II. Content Review
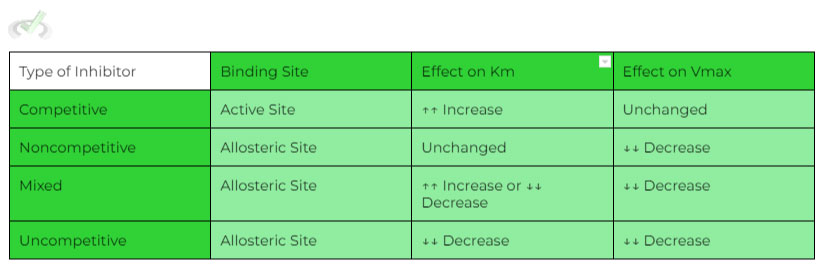
There are 4 types of reversible inhibition to be familiar with:
- Competitive Inhibition
- Noncompetitive Inhibition
- Mixed Inhibition
- Uncompetitive Inhibition
We’ll compare the different types of inhibition based on three factors: 1) how the inhibition changes the Km value, 2) how the inhibition changes the vmax value, & 3) what site the inhibition targets on the enzyme.
A. Competitive Inhibition
A. Site of Action: Active Site
B. Changes in Km: ↑↑ Increases
C. Changes in vmax: Stays Constant
i. Description:
Competitive inhibitors are also called substrate analogues because they are essentially mimicking the shape of enzyme substrates. As such, the competitive inhibitors work to block the active site. When the inhibitor occupies this active site, it creates an enzyme-inhibitor complex and the enzyzme cannot react until the inhibitor disassociates. The Km value increases, whereas, the Vmax remains constant.
ii. Lineweaver-Burk Graph:
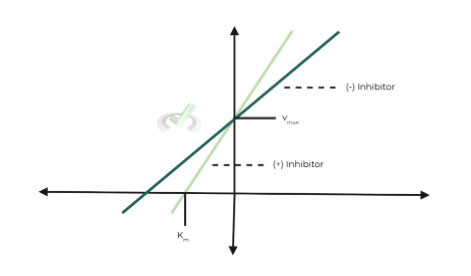
As you can see on the plot, Km is increased and Vmax is unaffected.
B. Noncompetitive Inhibition
A. Site of Action: Allosteric Site
B. Changes in Km: Stays Constant
C. Changes in vmax: ↓↓ Decreases
i. Description:
- Noncompetitive inhibitors alter enzyme function by binding to the allosteric site, which results in a conformational change in the enzyme’s shape.
- One important characteristic of noncompetitive inhibitors is that they bind w/ equal affinity to the free enzyme (i.e. no substrate present in the active site) and the enzyme-substrate complex (i.e. substrate occupying the active site).
ii. Lineweaver-Burk Graph:
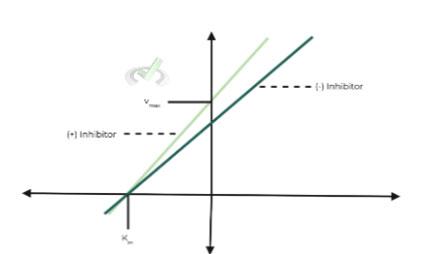
As you can see on the graph, Km appears to be unaffected, whereas Vmax has decreased.
C. Mixed Inhibition
A. Site of Action: Allosteric
B. Changes in Km: Depends
C. Changes in vmax: ↓↓ Decreases
i. Description:
Mixed inhibitor is when the inhibitor binds the enzyme at a location that is distant from the substrate binding site. Whether the Km increases or decreases depends on whether the mixed inhibitor binds with higher affinity to the free enzyme or the enzyme-substrate complex . It is called mixed inhibition as it is a mixture of competitive inhibition and uncompetitive inhibition.
- If inhibitor has higher affinity for free enzyme ⇒ Km increases
- If inhibitor has lower affinity for enzyme-substrate ⇒ Km decreases
ii. Lineweaver-Burk Graph:
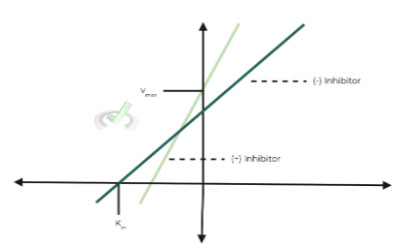
This is the lineweaver-burk graph for the instance when the inhibitor has a lower affinity for the enzyme-substrate as you can see the Km value has decreased.
D. Uncompetitive Inhibition
A. Site of Action: Allosteric Site
B. Changes in Km: ↓↓ Decreases
C. Changes in vmax: ↓↓ Decreases
i. Description:
- Uncompetitive inhibition strictly binds to only the enzyme-substrate complex as a part of its mechanism of action. Uncompetitive inhibition usually occurs in reactions with two or more substrates or products.
- An easy trick to spot uncompetitive inhibition when given a Lineweaver-Burk plot is that the lines representing the presence and lack of an inhibitor will always be parallel.
ii. Lineweaver-Burk Graph:
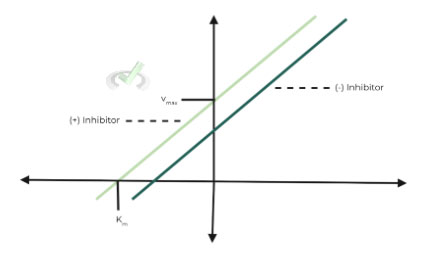
As you can see from the plot, both the Km and Vmax value have decreased to the same extent.
Refer to this chart for a quick overview of the different types of reversible inhibition!
III. Bridge/Overlap
Before we start, it would be a good idea to review the “Enzyme Kinetics” study article before tackling this study article. However, we’ll list out the most important concepts from the past article regardless as they may help you understand this section better.
Firstly, lets look back to this important rule of thumb to understand why the presence of an inhibitor causes a certain change in the line plotted in the Lineweaver-Burk graph.
Rule of Thumb:
The closer the intercept is to the origin (0,0), the LARGER the value of Km or vmax is (& vice versa)
It is important to remember that the intercepts represent the RECIPROCALs, so a LARGER value in the denominator will actually result in a LOWER value (& vice versa).
For example, noncompetitive inhibitors result in a decrease in the enzyme’s vmax. If you look at the Lineweaver-Burk plot, you see that the y-intercept for the line with the inhibitor is further from the origin. As such, it has a LOWER vmax value, as expected for a noncompetitive inhibitor.
You also might be wondering why certain inhibitors cause certain changes in the Km and the vmax values. For example, why do competitive inhibitors cause an increase in Km or noncompetitive inhibitors cause a decrease in vmax? It all comes back to understanding the structure of the enzyme as well as the meaning of both the Km & vmax values.
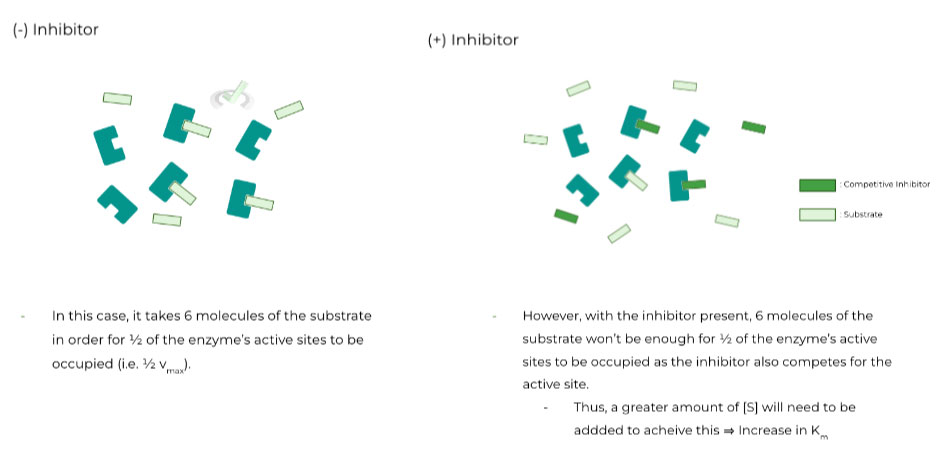
I. Competitive Inhibitors
As we saw previously, these types of inhibitors result in an increase in the enzyme’s Km value. In other words, the enzyme’s affinity for the substrate decreases ⇒ Why does this occur?
Competitive inhibitors are also known as substrate analogues, as they compete for the enzyme’s active binding site, resulting in decreased affinity for the original substrate.
Another way to think of it is that you’ll need to add a higher substrate concentration, [S], in order to have ½ of the enzyme’s active site occupied (i.e. ½ vmax)
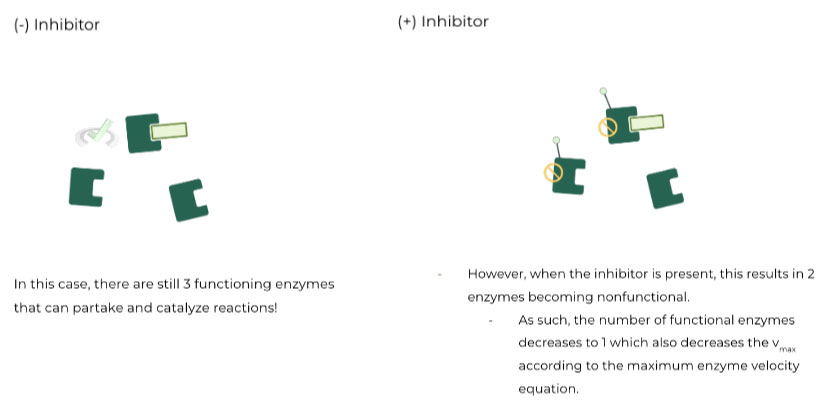
II. Noncompetitive Inhibitors
These types of inhibitors result in a decrease in the enzyme’s vmax. In other words, the enzyme’s maximum velocity rate decreases ⇒ Why does this occur?
When noncompetitive inhibitors bind to the enzyme’s allosteric site, they cause a conformational change resulting in the enzyme losing its function. This can occur in 2 ways:
The enzyme changes shape to where the active site is no longer accessible by the substrate ⇒ Occurs 50% of the time when inhibitor binds to free enzyme
The substrate is “locked” in the enzyme’s binding site, where it can no longer be released to form the product or bind another substrate ⇒ Occurs 50% of the time when inhibitor binds to enzyme-substrate complex
Now look back to the maximum enzyme velocity equation:
vmax = kcat [E]
Because the amount of functional enzyme, [E], decreases with noncompetitive inhibition, the vmax should also decrease based on the equation.
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
Enzyme Inhibition
Refers to how various molecules (most of the time drugs!) can inhibit enzyme function by changing the enzyme’s Km and/or vmax.
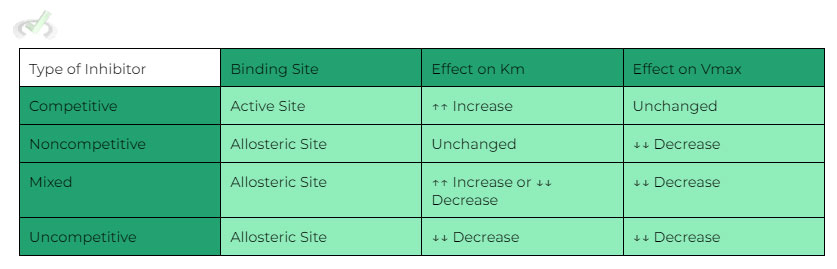
A. There are 4 types of reversible inhibition:
Competitive Inhibition
a. Site of Action: Active Site
b. Changes in Km: ↑↑ Increases
c. Changes in vmax: Stays Constant
B. Noncompetitive Inhibition
a. Site of Action: Allosteric Site
b. Changes in Km: Stays Constant
c. Changes in vmax: ↓↓ Decreases
C. Mixed Inhibition
a. Site of Action: Allosteric
b. Changes in Km: Depends
c. Changes in vmax: ↓↓ Decreases
D. Uncompetitive Inhibition
a. Site of Action: Allosteric Site
b. Changes in Km: ↓↓ Decreases
c. Changes in vmax: ↓↓ Decreases
Competitive Inhibitors ⇒ Why do they cause an increase in Km?
Competitive inhibitors act as substrate analogues, competing for the enzyme’s active site with the substrate.
As such, it will take a higher concentration of substrate, [S], to have ½ of the enzyme’s active sites occupied (i.e. ½ vmax).
Noncompetitive Inhibitors ⇒ Why do they cause a decrease in vmax?
Binding of noncompetitive inhibitors to the allosteric site results in a conformational change which results in a nonfunctional protein!
This results in a nonfunctional enzyme! Thus, according to the maximum enzyme velocity equation, vmax should also decrease as well!
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
Researchers are interested in understanding the enzyme kinetics when a newly developed inhibitor is present. When the plotting of the associated Lineweaver-Burk plot is finished, the researchers find that the lines representing the absence and present of the inhibitors share the same y-intercept. Which of the following best describes the inhibitors?
I. Competitive Inhibitor
II. Noncompetitive Inhibitor
III. Uncompetitive Inhibitor
A. I only
B. II only
C. III only
D. I or III
Ans. A.
To tackle this question, you must apply both enzyme kinetics & enzyme inhibition to arrive at your answer. Start by first understanding what the x and y intercepts mean:
X-intercept: Represents -1/Km
Y-intercept: Represents 1/vmax
Remember the rule of thumb!
The closer the intercept is to the origin, the larger the value of Km and vmax (& vice versa).
As stated in the question stem, the y-intercepts for the 2 lines are the same. As such, they should have the same vmax. All types of inhibition, except for competitive, result in a decrease in vmax (i.e. noncompetitive, mixed, uncompetitive). Thus, the only inhibition that can describe the inhibitor is competitive inhibition.
Sample Practice Question 2
When shown a Lineweaver-Burk plot showing an uncompetitive inof the following best describes how the slope of the line with the inhibitor compares to the line without the inhibitor?
A. Greater
B. Lesser
C. Same
D. None of the Above
Ans. C
Although this question may not directly involve enzyme inhibition, it’s always good to brush up on some math concepts as they may come up on the MCAT.
One easy way to identify an uncompetitive inhibition on a Lineweaver-Burk plot is that the lines representing the absence & presence of an inhibitor will be parallel.
Parallel lines will always have the same slope! The only thing that changes about the lines is how they’re shifted on the graph.
VI. Additional Links
Here are some additional links to related articles & additional resources that will hopefully be helpful to you all!







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
