I. What are the Key Reactions of Carbohydrates?
Being biochemical, organic molecules, carbohydrates have tons to offer in regards to the various reactions that they can participate in! Their unique structural properties as aldoses and ketoses allow for their involvement in these wide arrays of reactions!
While you might see some foreshadowing to organic chemistry, don’t be afraid! In fact, try to use this to your advantage as now you have a concrete example of how some of the organic chemistry concepts are applied in the context of biochemistry!
Though we’ll only stick to the high yield reactions that you’ll need to know, try and see what other reactions carbohydrates can participate in based on their structure! Again, it may be another great way to review and apply basic organic chemistry reactions to carbohydrates.
II. Reactions of Carbohydrates
There are 2 main carbohydrate reactions that we’ll cover: 1) Presence of Reducing Sugars and 2) Synthesis and Hydrolysis of Glycosidic Linkage.
A. Presence of Reducing Sugars
Let’s first outline the definition of reducing sugar! A reducing sugar refers to a sugar which contains a free anomeric carbon containing a hydroxyl group.
In other words, the anomeric carbon is not contained in a glycosidic bond. Likewise, a nonreducing sugar refers to a sugar which DOES NOT contain a free anomeric carbon.
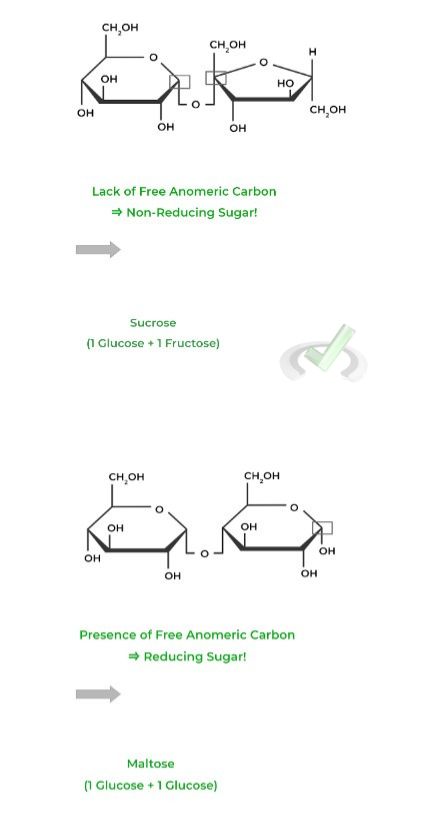
Notice how in sucrose, there is no free anomeric carbon because they both make up the 𝛂-1,2 glycosidic bond which is why it’s a nonreducing sugar!
Maltose, however, does still have a free anomeric carbon on the second glucose monomer, which is why it’s a reducing sugar.
I. Benedict’s and Tollen’s Tests
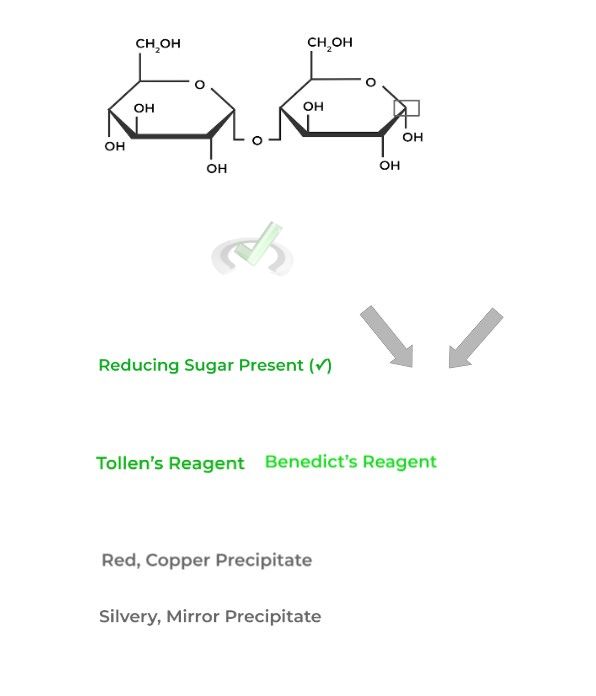
These tests are utilized in order to confirm the presence of a reducing sugar! They mainly differ over the result of a positive hit on a reducing sugar.
A positive hit of a reducing sugar utilizing a Benedict’s reagent will result in a red, copper precipitate emerging while one utilizing Tollen’s reagent will produce a silvery mirror!
B. Synthesis and Hydrolysis of Glycosidic Linkage
As we’ve mentioned in another article, disaccharides and polysaccharides are connected via glycosidic bonds! Additionally, it’s of great note to realize that these bonds are just one example of acetal and ketal formation!
Similarly, recall that the formation of these glycosidic bonds is an example of a dehydration synthesis, as a water
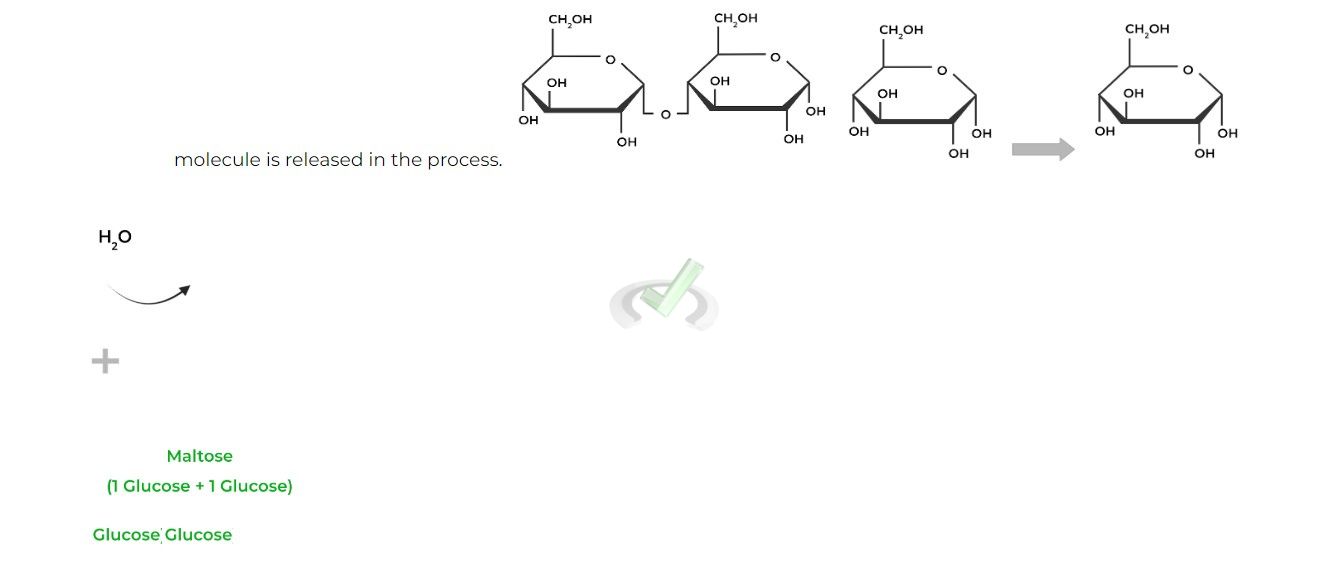
Notice how the C4 hydroxyl group on the right glucose will become the “-OR” group which will attack the C1 anomeric carbon, replacing the hydroxyl group eventually forming the acetal.
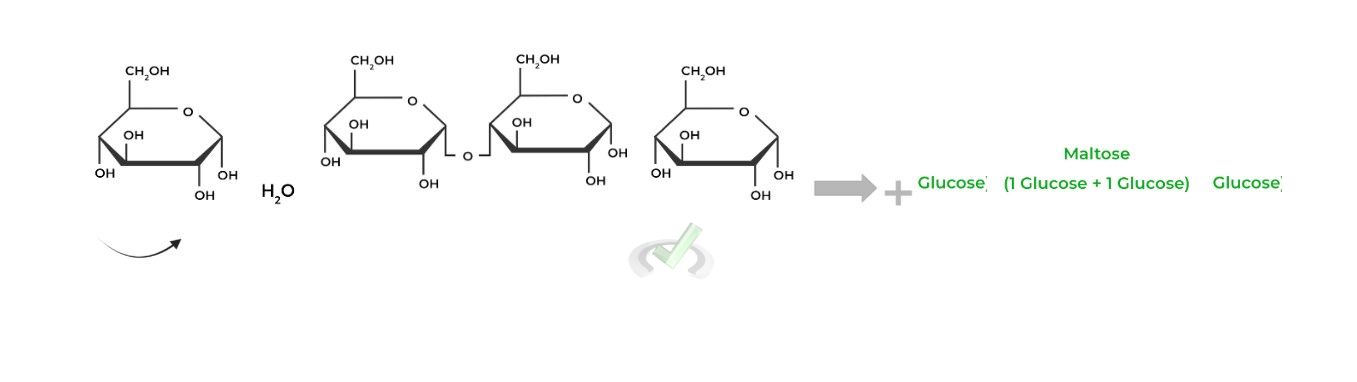
Contrarily, the breakage of these glycosidic bonds involves hydrolysis, as a water molecule acts as a nucleophile breaking the glycosidic bond.
III. Bridge/Overlap
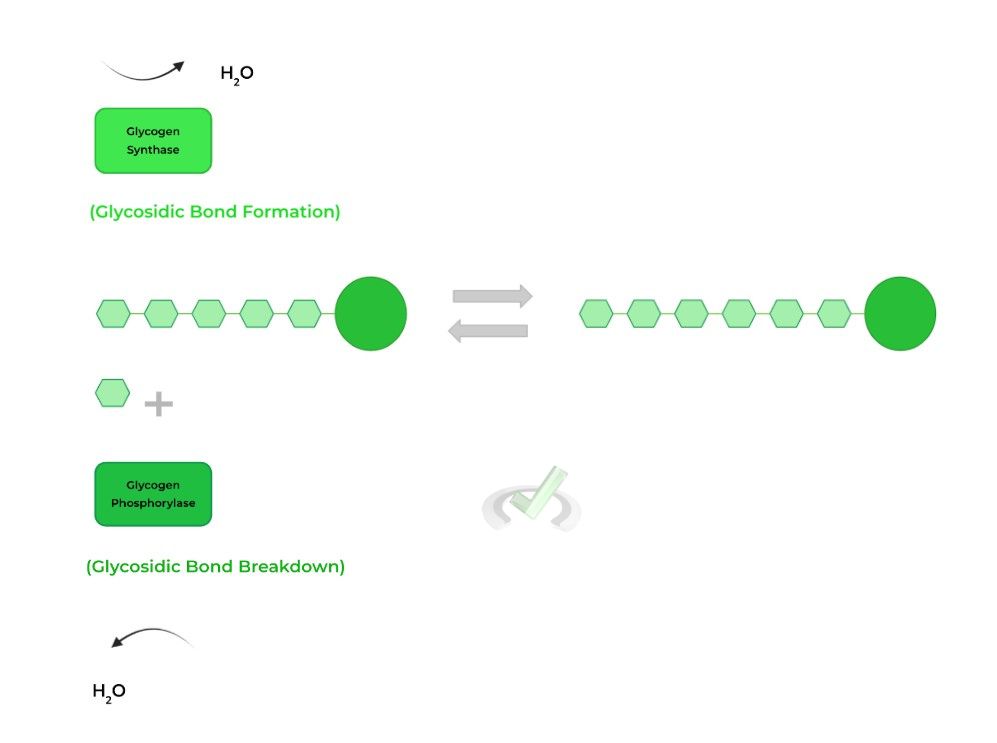
One of the best ways to review and learn the content you’ve learned here is to put them into a carbohydrate metabolism scenario. In this case, we’ll correlate how some of these reactions apply towards glycogen synthesis and breakdown!
I. Glycogen Synthesis and Breakdown
Though we have other articles which go into these topics in much more detail, we’ll cover the basics and how they relate to the reactions mentioned above!
Glycogenesis, simply known as glycogen synthesis, is primarily catalyzed by the rate limiting enzyme, glycogen synthase which catalyzes the formation of the glycosidic bonds!
Glycogenolysis, also known as glycogen breakdown, is catalyzed by glycogen phosphorylase which catalyzes the hydrolysis of the glycosidic bonds!
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Presence of Reducing Sugars
A reducing sugar is one that contains a free anomeric carbon with a hydroxyl while a nonreducing sugar is one that is lacking! In other words, a reducing sugar is one where the anomeric carbon is not within the glycosidic bond.
I. Benedict’s and Tollen’s Tests
These are tests that use reagents of the same name to determine the presence of a reducing sugar, where positive tests indicate the presence of a reducing sugar!
A positive Benedict’s test results in a red, copper precipitate forming while a Tollen’s test results in a silvery, mirror precipitate.
B. Synthesis and Hydrolysis of Glycosidic Linkages
The formation of glycosidic linkages actually has its basis in organic chemistry, as they result in the formation of acetals and ketals, formed via a dehydration synthesis!
Essentially, an hydroxyl group on one sugar will become the additional “-OR” group in the acetal and/or ketal.
Likewise, the breakdown of glycosidic linkages involves hydrolysis, similar to other reactions, where the water molecule acts as the nucleophile.
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
Given the structure of lactose below, which of the following best details what will occur if we conduct a Tollen’s test on lactose?
A. Positive, Red Copper Precipitate Forms
B. Positive, Silvery Mirror Precipitate Forms
C. Negative, Red Copper Precipitate DOES NOT Form
D. Negative, Silvery Mirror Precipitate DOES NOT Form
Ans. B
Galactose is a reducing sugar because it still has a free anomeric carbon shown on the right glucose molecule! Thus, when it undergoes a Tollen’s test, the test will be positive resulting in the production of a silvery, mirror precipitate.
Sample Practice Question 2
Lactase is an enzyme responsible for the digestion of lactose. Which type of reaction will occur and what functional group will emerge as a result?
A. Dehydration Synthesis, Acetal
B. Dehydration Synthesis, Hemiacetal
C. Hydrolysis, Acetal
D. Hydrolysis, Hemiacetal
Ans. D
In order to allow for the digestion of lactose, it must be broken down into its monomeric subunits via hydrolysis. As such, the acetal functional group that was formed via the glycosidic bond will be broken, resulting in a hemiacetal group reemerging.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot