Carbohydrates are a very crucial part of your diet and physiological functioning. As you’ll see, these simple and complex sugars are a crucial energy source for the body to power the various cellular processes that keep us functioning!
This guide will introduce carbohydrate structure, some key terms, and definitions you should remember as you prepare for the MCAT.
Let’s get started!
Carbohydrate Structure on the MCAT: What Do You Need to Know?
Carbohydrate structure is covered in the Biochemistry section of the MCAT.
Introductory biochemistry accounts for 25% of the Biological and Biochemical Foundations of Living Systems section (Bio/Biochem) and 25% of the Chemical and Physical Foundations of Biological Systems section (Chem/Phys).
It’s hard to predict the exact number of questions about carbohydrates that will appear on the MCAT. However, you can expect it to appear in both the Bio/Biochem and Chem/Phys sections.
Important Sub-Topics – Carbohydrate Structure
We cannot stress enough the importance of carbohydrates during MCAT prep since they are closely related to basic organic chemistry, another important MCAT topic!
Carbohydrates can be complicated, so let’s break them down into important sub-topics you can concentrate on.
1. Carbohydrates: Structural Description
Carbohydrates are biomolecules consisting of oxygen, hydrogen, and carbon atoms. Carbohydrates encompass all sugars, both simple and complex sugars. Simple sugars include monosaccharides and disaccharides while complex sugars include oligosaccharides and polysaccharides.
As for carbon numbering, carbohydrates follow the same rules as carbons in organic chemistry!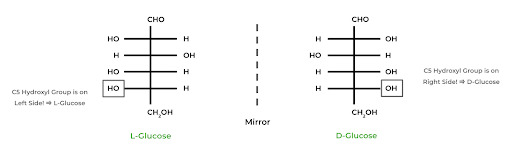
There are two main ways carbohydrates can be represented on paper: the Fischer Projection and the Hawthorne Projection, which are used for depicting sugars in a planar and cyclic way, respectively.
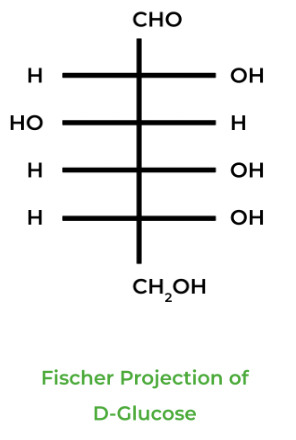
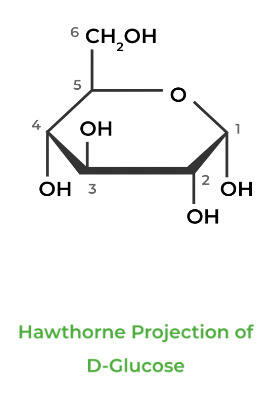
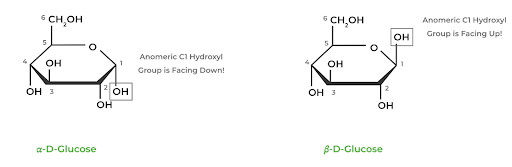
In carbohydrate biochemistry, there are two important stereochemical topics: enantiomers in D and L Fisher projections and anomeric epimers in Hawthorne projections.
Carbohydrate sugars come in enantiomeric pairs also known as mirrored pairs, indicated by the D or L in front of the sugar name. The D indicates that the hydroxyl group on the highest numbered chiral carbon is ON THE RIGHT of the Fisher projection, while the L indicates that it’s ON THE LEFT.
Anomeric epimers are stereoisomers differing only in the position of one stereocenter. For example, the alpha anomer of D-glucose has the anomeric hydroxyl group facing DOWN while it’s facing UP in the beta anomer.
Full Study Notes : Carbohydrates: Structural Description on the MCAT
For more in-depth content review on structural description, check out these detailed lesson notes created by top MCAT scorers.
2. Carbohydrates: Commonly Tested Sugars
We’ll break down the commonly tested carbohydrates into monosaccharides, disaccharides, and polysaccharides.
1. Monosaccharides
The simplest sugar formation; it is the building block for carbohydrates. Monosaccharides can be linked together to form disaccharides and polysaccharides. Examples include glucose, fructose, and galactose.
2. Disaccharides
These are composed of two monosaccharide subunits. Examples include sucrose, lactose, and maltose.
3. Polysaccharides
These are composed of many monosaccharide subunits. Examples include glycogen, starch, and cellulose.
Full Study Notes : Carbohydrates : Commonly Tested Sugars on the MCAT
For more in-depth content review on commonly tested sugars, check out these detailed lesson notes created by top MCAT scorers.
3. Carbohydrates: Key Reactions
Carbohydrates' unique structural properties allow for their involvement in many different reactions!
There are two main carbohydrate reactions that we’ll cover: the presence of reducing sugars and the synthesis and hydrolysis of glycosidic linkages.
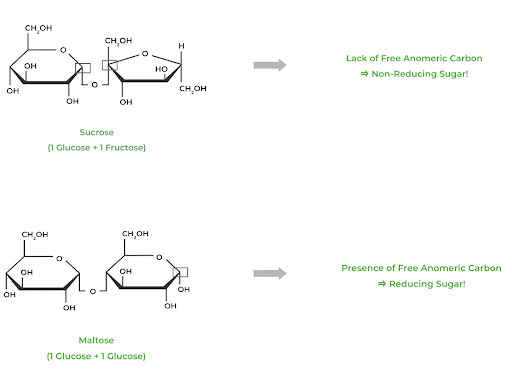
Reducing sugars exist when the anomeric carbon is free, meaning it is not involved in a glycosidic bond. Likewise, a nonreducing sugar refers to a sugar that DOES NOT contain a free anomeric carbon. You can detect the presence of a reducing sugar with Tollen’s or Benedict’s tests.
The second main reaction is the hydrolysis of glycosidic linkages. The formation of these linkages results in the formation of acetals and ketals, formed via a dehydration synthesis! Essentially, a hydroxyl group on one sugar will become the additional “-OR” group in the acetal and/or ketal.
Likewise, the breakdown of glycosidic linkages involves hydrolysis, similar to other reactions, where the water molecule acts as the nucleophile.
Full Study Notes : Carbohydrates: Key Reactions on the MCAT
For more in-depth content review on carbohydrates key reaction, check out these detailed lesson notes created by top MCAT scorers.
Key Terms and Definitions – Carbohydrates
Here are some of the more important key terms and definitions to remember for this general guide to carbohydrates!
Term | Definition |
|---|---|
Monosaccharides | The most basic unit of carbohydrates. |
Disaccharides | The carbohydrate formed when two monosaccharides are bound by a glycosidic linkage. |
Polysaccharides | The carbohydrate formed when more than two monosaccharides are bound by a glycosidic linkage. |
Glycosidic linkage | A covalent bond that joins a carbohydrate group to another molecule. |
Epimers | A type of stereoisomer that differs in configuration at the anomeric carbon. |
Anomers (type of epimer) | A type of stereoisomer that differs in configuration at the hemiacetal or acetal carbon. |
Additional FAQs – Carbohydrates on the MCAT
What are the 4 structures of carbohydrates?
Should you memorize carbohydrate structure for the MCAT?
How do I remember the carbohydrate sugars?
What is the basic structure of carbohydrates?
Additional Reading Links – Study Notes for Carbohydrates Structure on the MCAT
For more in-depth content review about carbohydrates structure on the MCAT, check out these detailed lesson notes created by top MCAT scorers!
Additional Reading: Biochemistry Subjects on the MCAT:
- Biological Membranes on the MCAT
- Carbohydrate Metabolism on the MCAT
- Amino Acids Peptides Proteins on the MCAT
- DNA and RNA on the MCAT
- Enzymes on the MCAT
- Lipids and Lipid Metabolism on the MCAT
- Non-Enzymatic Proteins on the MCAT
- Regulation of Metabolism on the MCAT
- Biotechnology on the MCAT
- Bioenergetics on the MCAT







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot