Randomized Control Trials (RCTs) are tests used in research to see how well new treatments work. This guide explains RCTs, their work, their main parts, common challenges, and how they connect to other science and medicine topics.
I. What Are Randomized Control Trials?
RCTs are the best way to test new treatments. Here’s why they are special:
A. Definition and Purpose
An RCT is a test where participants are randomly put into two groups. One group gets the treatment being tested, while the other group does not. This other group is called the control group. The goal is to see if the treatment works better than no treatment or another treatment.
B. Importance of Randomization

Randomization means assigning participants to the treatment or control group by chance, like flipping a coin. This helps prevent bias, ensuring that differences in results are due to the treatment itself and not other factors. For example, it avoids situations where one group might have more people with a specific health condition, which could skew the results.
C. Control Groups: The Comparison

The control group provides a standard to compare against the treatment group. This group might receive a placebo, which is a harmless, fake treatment, or no treatment at all. This comparison helps researchers see if the treatment has a real effect beyond what might happen by chance or expectation.
II. The RCT Process: How It Works
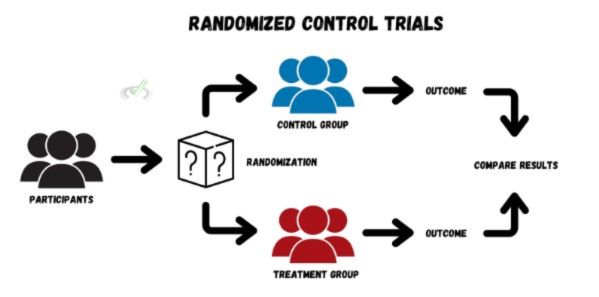
RCTs follow a structured process to make sure the findings are reliable. Here’s how they typically work:
A. Designing the Study
The first step is designing the study. Researchers decide on the specific question they want to answer and how they will measure the results. They also set criteria for who can participate, such as age, health status, or other factors.
B. Participant Recruitment and Randomization
Participants are recruited based on the set criteria. Once selected, they are randomly assigned to either the treatment group or the control group. Randomization helps ensure that the groups are similar regarding key characteristics, making the comparison fair.
C. Implementing the Intervention
The treatment group receives the intervention, such as a new drug, therapy, or procedure. The control group might receive a placebo or the usual treatment. The intervention is the main variable being tested to see if it has the intended effect.
D. Monitoring and Data Collection
During the study, researchers monitor both groups and collect data. They measure specific outcomes, like symptom improvement or health marker changes. This data is critical for analyzing the effectiveness of the treatment.
E. Analyzing the Results
After collecting the data, researchers analyze it to see if there are meaningful differences between the groups. They use statistical methods to determine if the results are significant or could have happened by chance.
III. Key Components of RCTs
To understand RCTs, it's important to know about their main components:
A. Blinding
Blinding means keeping the participants and sometimes the researchers unaware of who is in the treatment or control group. This prevents bias. There are different types of blinding:
- Single-blind: Only the participants don’t know which group they are in.
- Double-blind: Both the participants and the researchers don’t know.
- Triple-blind: Even those analyzing the data don’t know. This level of blinding is very effective in preventing biases in data interpretation.
B. Sample Size
The number of participants in an RCT, known as the sample size, is crucial. A larger sample size usually leads to more reliable results because it reduces the chance that the results are due to random variations. It helps to ensure that the findings apply to a larger population.
C. Outcome Measures
Outcome measures are the specific things researchers are looking to improve or change with the treatment. These could include symptom relief, cure rates, or other health indicators. These measures must be clearly defined and consistently used throughout the study.
IV. Challenges in Conducting RCTs
Even though RCTs are highly valued, they come with challenges:
A. Ethical Concerns
Conducting RCTs involves ethical considerations. For example, it might be unethical to withhold a potentially life-saving treatment from the control group. Researchers must ensure that participants give informed consent, meaning they fully understand the risks and benefits of participating in the trial.
B. Cost and Time
RCTs can be expensive and take a long time to complete. They require a lot of resources for planning, recruiting participants, and collecting data. This can limit the number of RCTs conducted, especially in underfunded research areas.
C. Generalizability
The results of an RCT may not always apply to the general population if the study participants are not diverse. For example, a study with mostly young, healthy participants may not show how a treatment works in older or sicker individuals. This is known as a limitation in external validity.
V. Bridge/Overlap
RCTs connect with many other areas of science and medicine. They are especially important in the following fields:
A. Evidence-Based Medicine
Evidence-based medicine relies on the best available evidence, often from RCTs, to make decisions about patient care. This approach helps ensure safe and effective treatments, improving healthcare outcomes.
B. Public Health and Policy
Findings from RCTs can influence public health policies. For example, if an RCT shows that a new vaccine is safe and effective, health authorities might recommend it to the population. This can lead to widespread health interventions and improvements.
C. Clinical Guidelines
Many clinical guidelines are based on evidence from RCTs. These guidelines help doctors and other healthcare providers choose the best treatments for their patients. Following these guidelines helps standardize care and improve patient outcomes.
D. Further Research and Innovation
Results from RCTs often lead to further research. For example, if an RCT shows that a new drug works, scientists may conduct more studies to understand how it works or to test it in different populations. This process helps build scientific knowledge and leads to innovations.
VI. Wrap-Up/Key Terms
RCTs are a key method for testing new treatments and interventions. They involve randomization, control groups, and careful data analysis to provide reliable results.
Key Terms
- Randomization: Assigning participants to groups by chance to avoid bias.
- Control Group: A group that does not receive the treatment is used for comparison.
- Blinding: Keeping participants and researchers unaware of group assignments to prevent bias.
- Outcome Measures: Specific indicators used to assess the effects of the treatment.
- Evidence-Based Medicine: Using the best available research to make medical decisions.
VII. Practice
Sample Practice Question 1
What is the purpose of randomization in an RCT?
A. To ensure all participants receive the treatment
B. To avoid bias, characteristics should be evenly distributed between groups
C. To select the best participants for the study
D. To increase the cost of the study
Ans. B
Randomization ensures that the groups are similar in key characteristics, making the comparison fair and unbiased.
Sample Practice Question 2
Why might a control group receive a placebo in an RCT?
A. To trick participants
B. To prevent them from knowing they are in the control group
C. To provide a baseline for comparing the effects of the treatment
D. To reduce the cost of the study
Ans. C
The placebo allows researchers to see if the treatment has a real effect beyond what participants might experience by expecting to receive treatment.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot