Functional groups are specific groups of atoms within molecules. They give the molecule certain properties and behaviors. The order of these groups is essential in the IUPAC naming system.
This system helps us name organic compounds the same way every time. By learning this order, we can name even the most complex molecules correctly.
I. Key Functional Groups and Their Properties
Alkanes
Alkanes are the simplest organic compounds. They consist only of carbon and hydrogen atoms connected by single bonds. In naming, alkanes are the starting point. Other functional groups take priority over them.
Alkenes and Alkynes
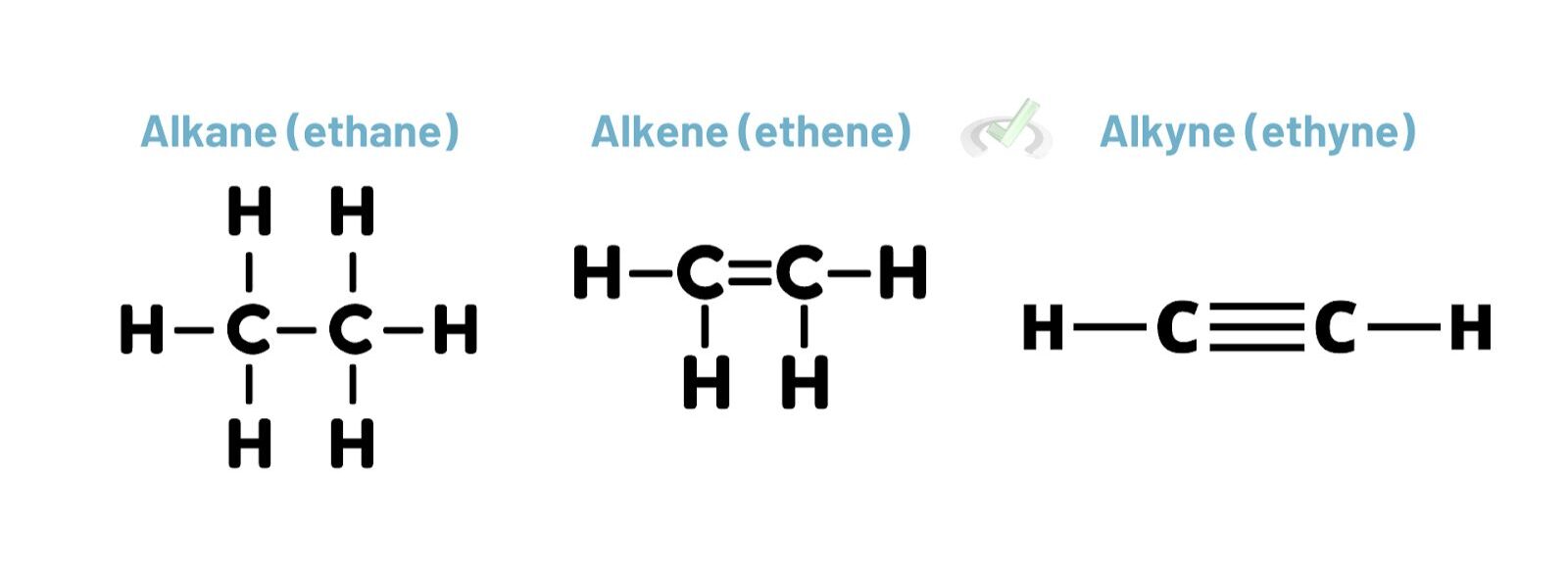
Alkenes have double bonds, and alkynes have triple bonds. These unsaturated hydrocarbons have higher priority than alkanes. However, they are usually lower than more complex functional groups
Alcohols
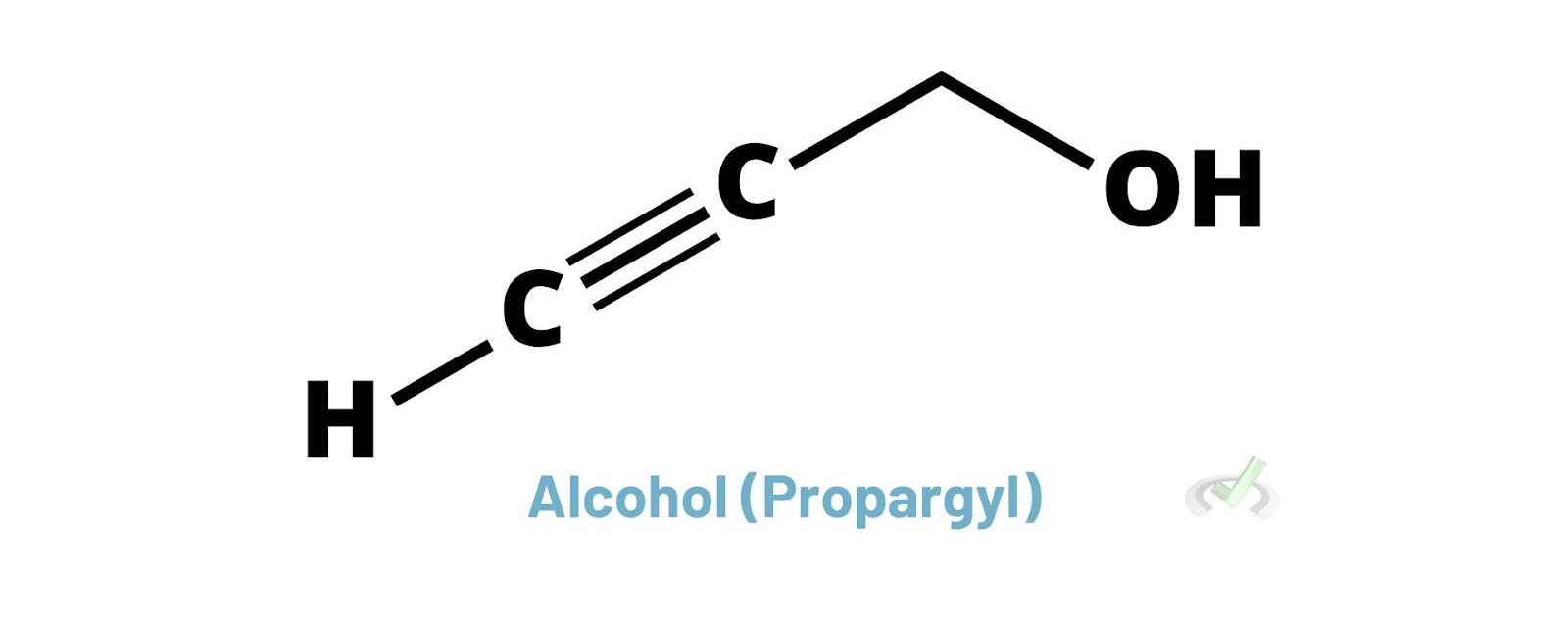
Alcohols have an -OH group attached to a carbon atom. This group has higher priority than alkanes, alkenes, and alkynes. When naming compounds with alcohols, we use the suffix "-ol."
Aldehydes and Ketones
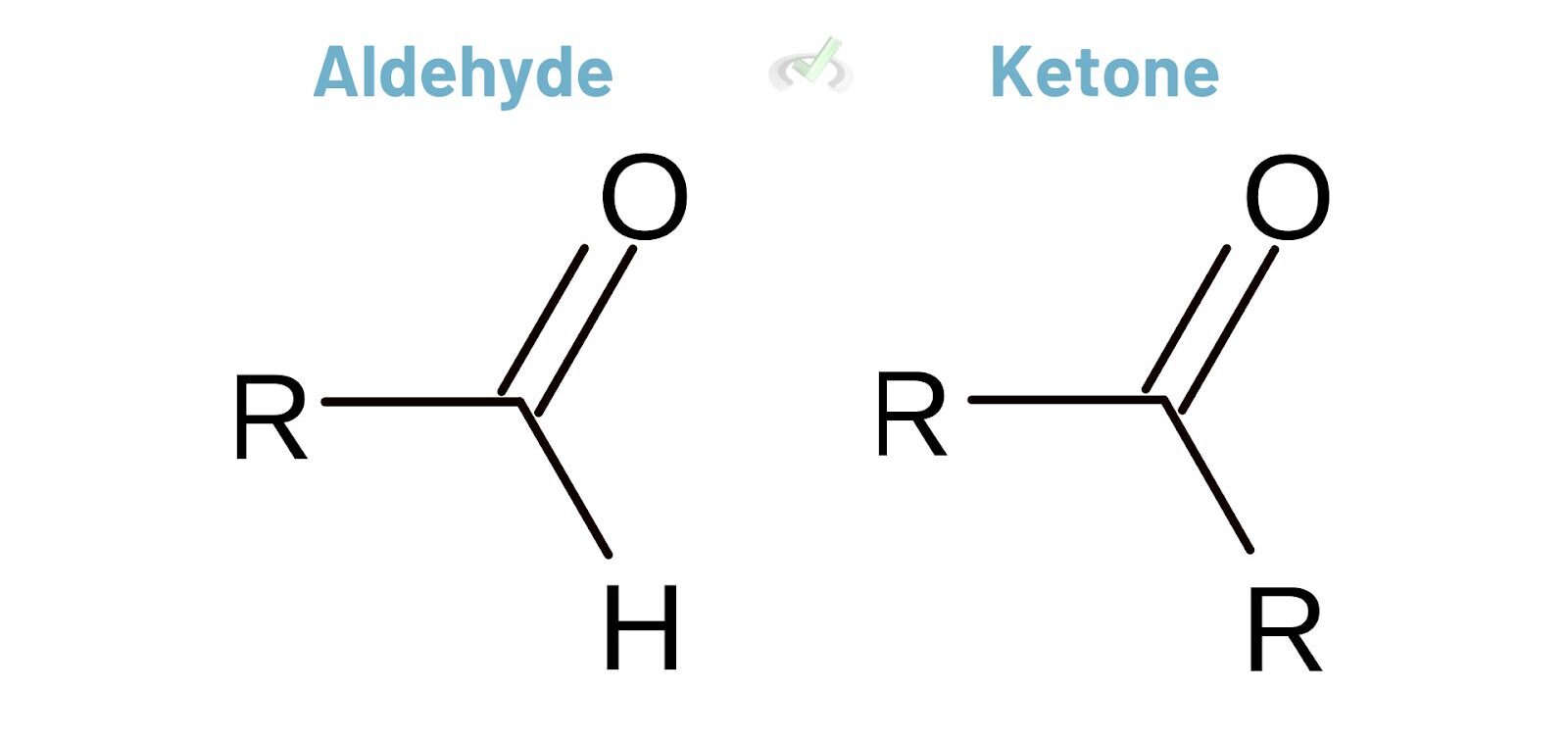
Aldehydes and ketones have a carbonyl group (C=O). Aldehydes have this group at the end of a carbon chain. Ketones have it within the chain. Aldehydes generally have higher priority than ketones in naming.
Carboxylic Acids
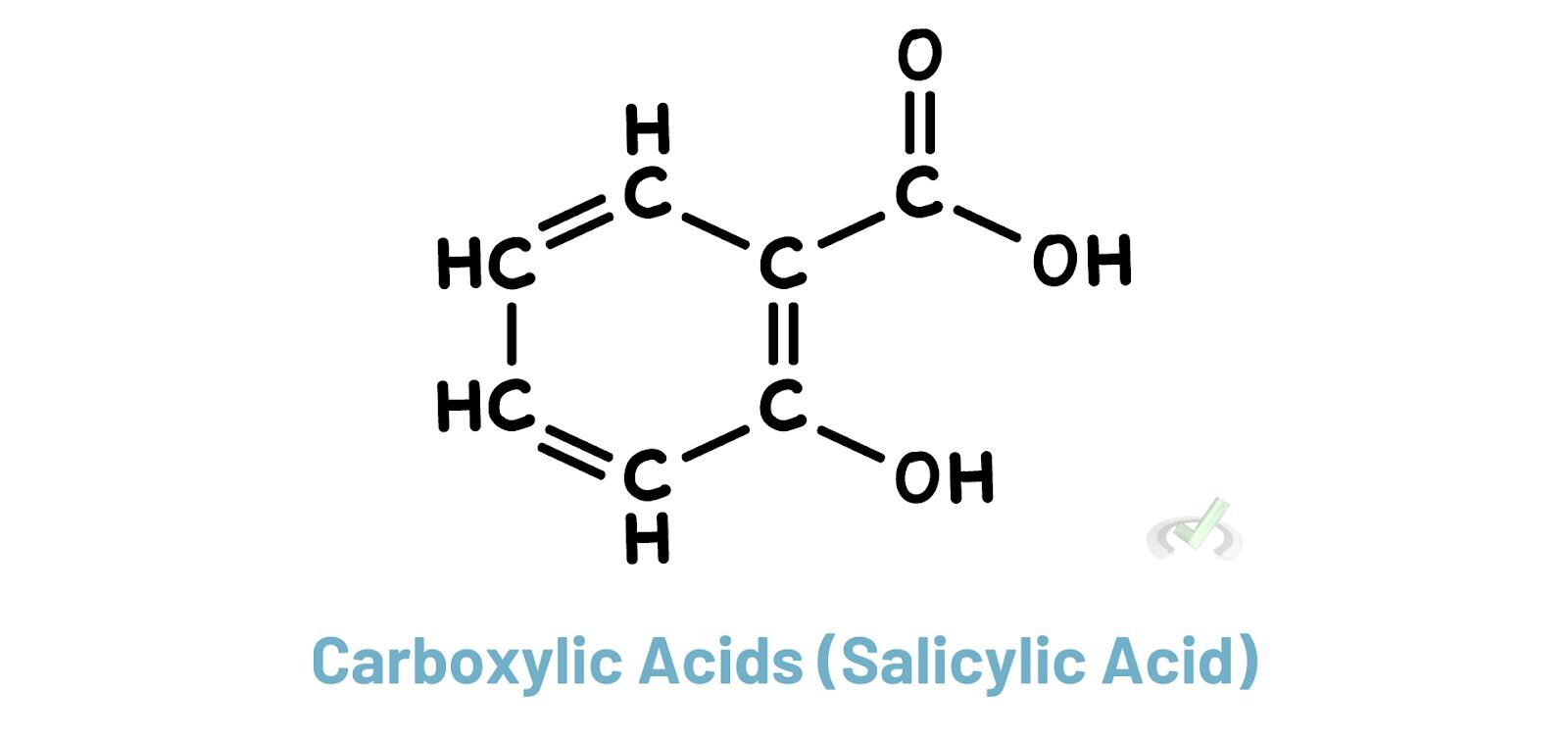
Carboxylic acids have a -COOH group. They are among the highest-priority functional groups. They influence the naming of compounds with the suffix "-oic acid."
Esters, Amides, and Anhydrides
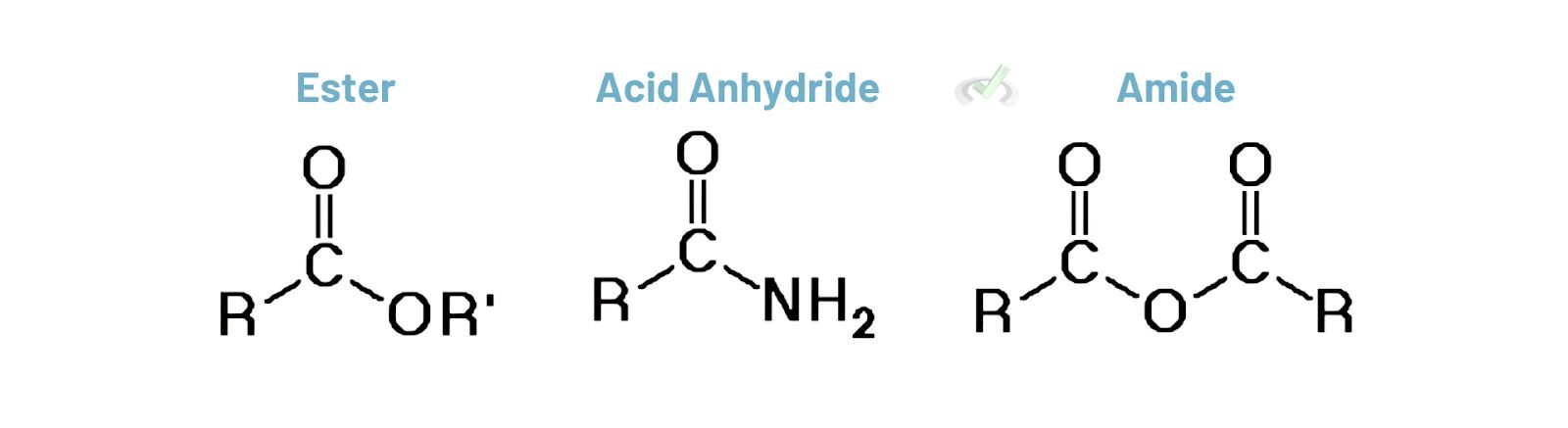
Esters (-COOR), amides (-CONH2), and anhydrides (two -COOH groups joined by an oxygen atom) also have high priority. These groups often appear in more complex molecules and influence the base name of the compound.
Amines and Ethers
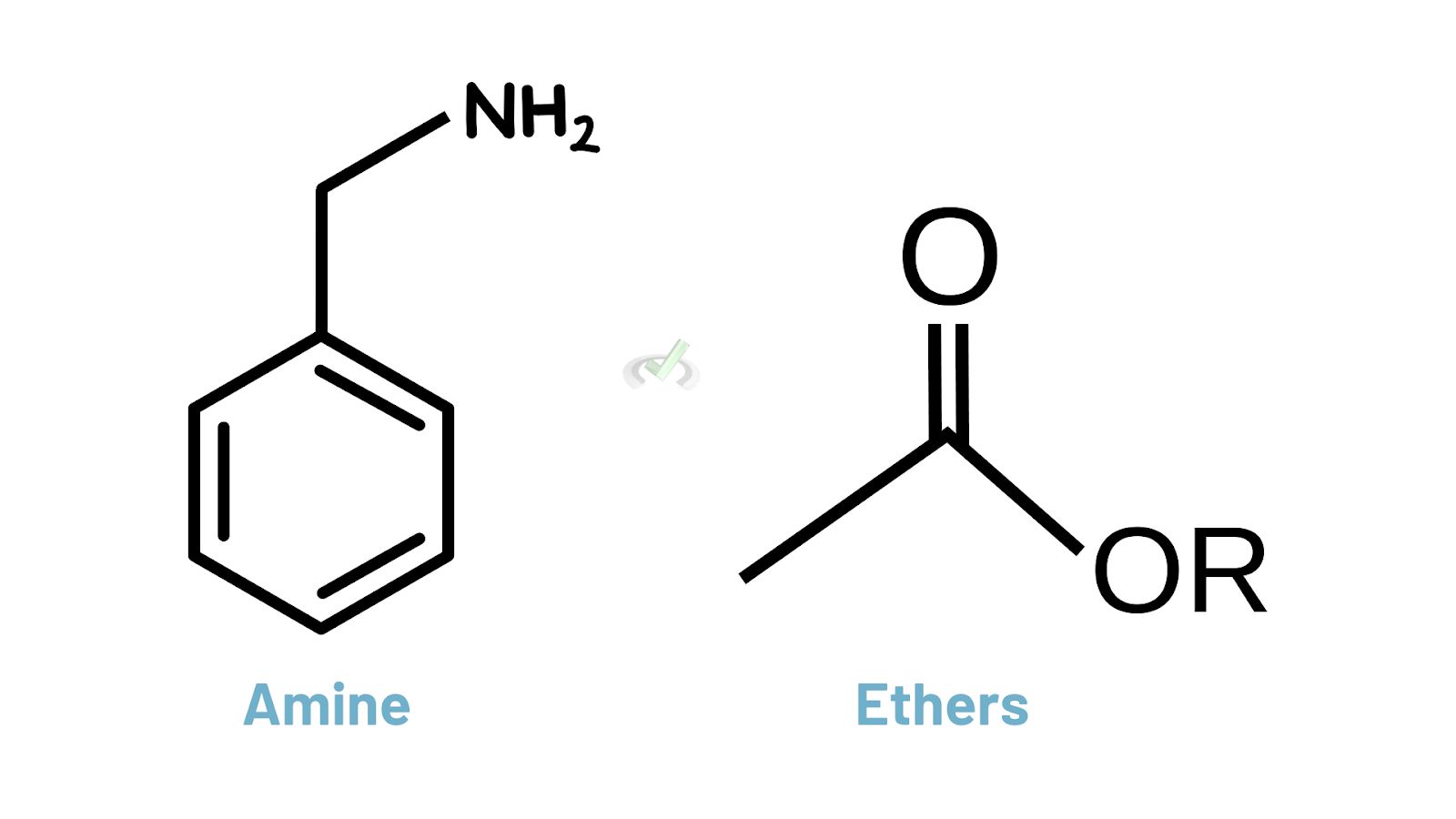
Amines (-NH2) and ethers (R-O-R) have a lower priority compared to alcohols, aldehydes, and carboxylic acids. But they still play a significant role in the structure and reactivity of organic molecules.
Thiols and Nitriles
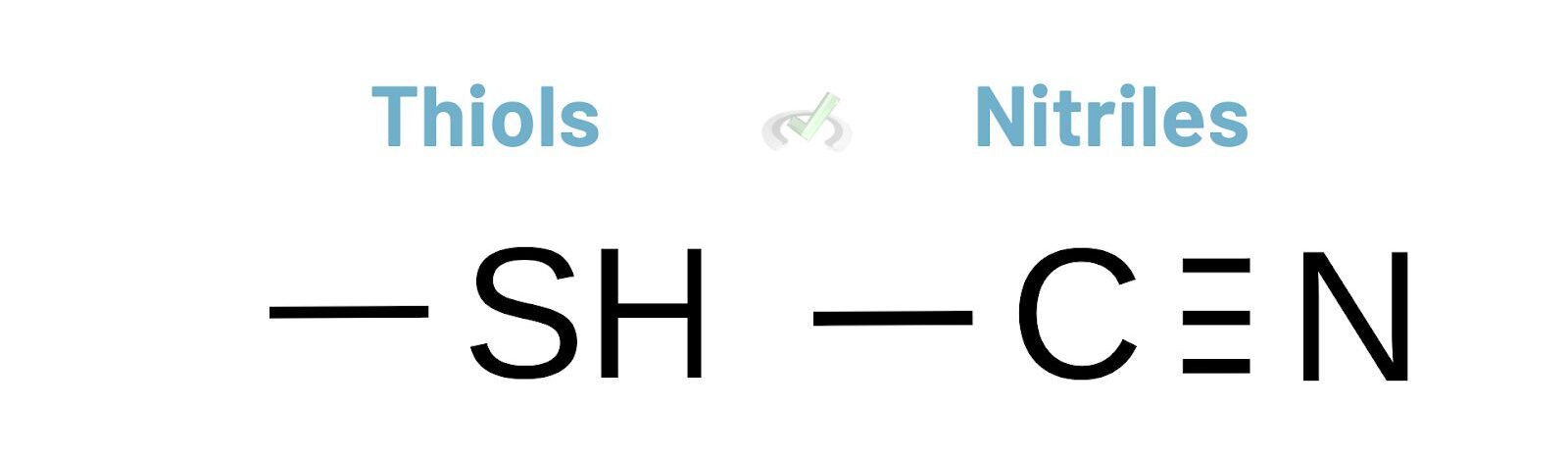
Thiols have an -SH group, and nitriles have a -C≡N group. These groups are important in various chemical reactions and have their own priority levels in naming.
II. How Priority Ordering Works
General Rules
The priority of functional groups depends on their reactivity and the number of bonds to heteroatoms (atoms other than carbon and hydrogen). More reactive groups with multiple bonds to heteroatoms generally have higher priority. The IUPAC rules state that the highest priority group sets the suffix of the compound name. Other groups are treated as prefixes.
Example: Naming a Complex Molecule
Consider a molecule with an alcohol, an aldehyde, and a carboxylic acid group. The carboxylic acid group takes the highest priority and determines the suffix of the name. The alcohol and aldehyde groups are named as substituents with the prefixes "hydroxy-" and "oxo-," respectively.
III. Practical Applications of Priority Ordering
Naming Organic Compounds
Priority ordering is used to systematically name organic compounds. By following IUPAC rules, chemists can name compounds in a way that clearly describes their structure and functional groups.
Predicting Reactivity
Understanding the priority of functional groups helps predict how different parts of a molecule will react. This knowledge is crucial in organic synthesis and understanding reaction mechanisms.
Analyzing Spectroscopic Data
Spectroscopic techniques like NMR and IR spectroscopy rely on the identification of functional groups. Knowing their priority can help interpret spectra and identify unknown compounds.
IV. Advanced Concepts in Functional Group Priority
Polyfunctional Compounds
Compounds with multiple functional groups present challenges in naming. The IUPAC rules provide guidelines for naming such compounds, ensuring clarity and consistency.
Stereochemistry and Priority
Stereochemistry involves the spatial arrangement of atoms in molecules. The Cahn-Ingold-Prelog priority rules extend to stereoisomers, helping determine the configuration of chiral centers in molecules.
Biological Molecules
Biologically active molecules often contain multiple functional groups. Understanding the priority of these groups is essential for naming these compounds. It also helps us understand their biological activity, interactions, and roles in biochemical pathways.
V. The Broader Impact of Functional Group Priority
In Organic Synthesis
Knowing the priority of functional groups is crucial for designing synthetic pathways for complex molecules. It allows chemists to plan reactions that introduce or modify specific functional groups in a controlled manner.
In Pharmaceutical Chemistry
Drug molecules often contain multiple functional groups that affect their activity and metabolism. Proper naming and understanding of these groups are crucial in drug development and regulatory approval processes.
In Material Science
Functional groups influence the properties of materials like polymers and nanomaterials. Knowing their priority helps in designing materials with specific properties for various applications.
VI. Wrap-Up and Key Terms
Understanding the priority ordering of functional groups is essential for mastering organic chemistry naming. Let's review the key terms and concepts:
Key Terms:
Functional Group: A specific group of atoms within a molecule that has characteristic properties.
IUPAC Nomenclature: A standardized system for naming organic compounds.
Priority Order: The hierarchy of functional groups that determines the suffix and prefix in compound names.
Alkane: A hydrocarbon with single bonds.
Alkene: A hydrocarbon with double bonds.
Alkyne: A hydrocarbon with triple bonds.
Alcohol: A compound with an -OH group.
Aldehyde: A compound with a carbonyl group at the end of a carbon chain.
Ketone: A compound with a carbonyl group within a carbon chain.
Carboxylic Acid: A compound with a -COOH group.
Ester: A compound with a -COOR group.
Amide: A compound with a -CONH2 group.
Anhydride: A compound with two -COOH groups joined by an oxygen atom.
Amine: A compound with an -NH2 group.
Ether: A compound with an R-O-R group.
Thiol: A compound with an -SH group.
Nitrile: A compound with a -C≡N group.
VII. Practice Questions
Sample Practice Question 1
Which functional group has the highest priority in nomenclature?
A) Alcohol
B) Ketone
C) Carboxylic Acid
D) Amine
Ans. C
Carboxylic acids have the highest priority among the given options. They dictate the suffix in the compound's name. Alcohols, ketones, and amines have lower priority.
Sample Practice Question 2
How would you name a compound with both an alcohol and a ketone group?
A) The ketone determines the suffix, and the alcohol is named with a prefix.
B) The alcohol determines the suffix, and the ketone is named with a prefix.
C) Both groups are named with prefixes.
D) Neither group affects the naming.
Ans. A
Ketones have higher priority than alcohols, so the ketone group determines the suffix, while the alcohol group is named as a substituent with the prefix "hydroxy-."



 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
























