Our body can make amino acids to build proteins necessary for our daily lives. Every living thing has its own complicated mechanisms to produce amino acids. Scientists sometimes need amino acids to develop new drugs, antibodies, and for other purposes. Luckily, scientists have found ways to make amino acids inside the laboratory.
In this article, we’ll talk about the two laboratory methods of making amino acids. As mentioned in our introductory article, you do not have to focus on the mechanism itself. The key here is to familiarize yourself with the steps and the important compounds involved.
Recall
Chemists tend to draw molecules that have a mirror image using Fischer projection. Having a mirror image or “chirality” is important because a compound can take on the structure of its mirror image and bear the same atoms, but it will have different ways of interacting with other molecules.
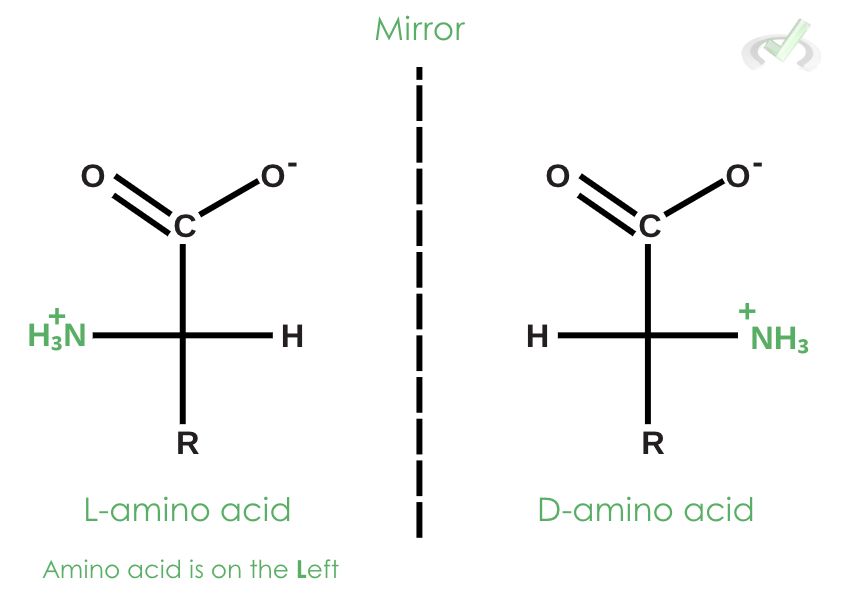
Fischer projections allow us to see how the three-dimensional structure of amino acids works. It’s important to remember that all amino acids in animals have L-configured amino acids, and D-amino acids occur in specialized structures. The L-configuration and D-configuration are called enantiomers since they are mirror images of each other.
I. Strecker Synthesis
Strecker synthesis uses ammonium and cyanide to make amino acids. The amino acids produced from this method are an equal mix of L and D-amino acids, meaning they are racemic. Most naturally occurring amino acids have an L-configuration. Since amino acids produced from this process are racemic, they may include inactive amino acids. Still, it effectively produces amino acids that may be naturally occurring or synthetic.
Here’s the three-step mechanism for Strecker Synthesis.
1. Imine Formation
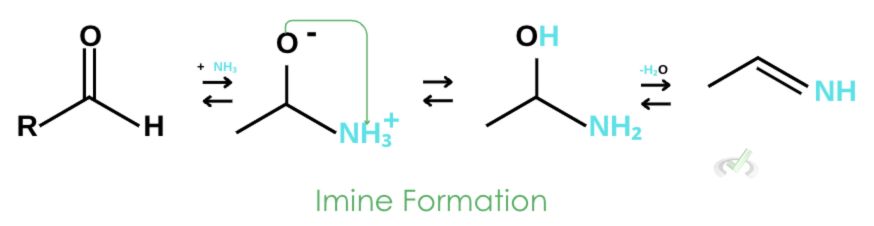
Ammonia attacks the carbonyl carbon. This breaks the double bond in the carbonyl oxygen, leaving it with a negative charge. Ammonia attaches itself as hydrogen is ejected. Oxygen protonates from ammonia and continues to do so until water is removed as a leaving group, forming an imine.
2. Cyanide Addition
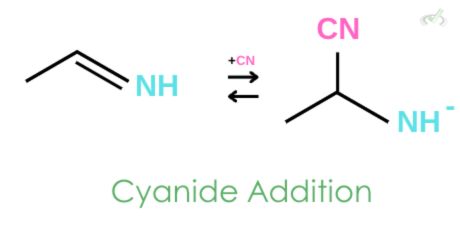
A cyanide salt is added to the imine, forming a bond with carbon and leaving the amine with a negative charge.
3. Acid Hydrolysis
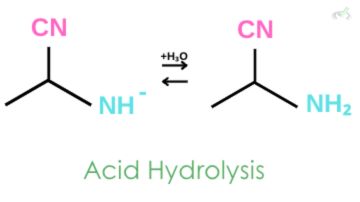
Hydronium ion donates a proton to the negative amine, and an alpha-aminonitrile is formed.
II. Gabriel Synthesis
Gabriel synthesis (sometimes called Gabriel-malonic ester synthesis) is another method used in acid synthesis. This synthesis produces a primary amine using phthalimide, which can later be used to make amino acid, which is a key reagent.
1. Deprotonation by a strong base
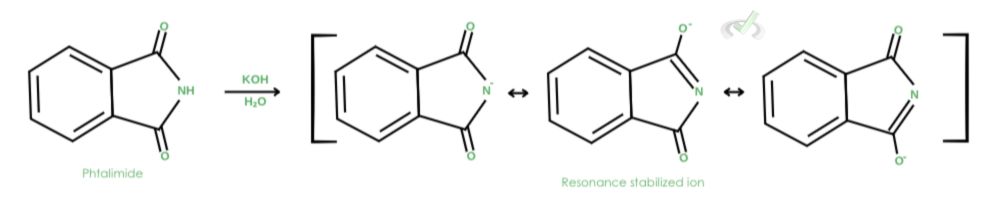
In this example, a strong base in the form of potassium hydroxide (KOH) deprotonates phthalimide, forming a resonance-stabilized anion.
2. SN2 Reaction with alkyl halide
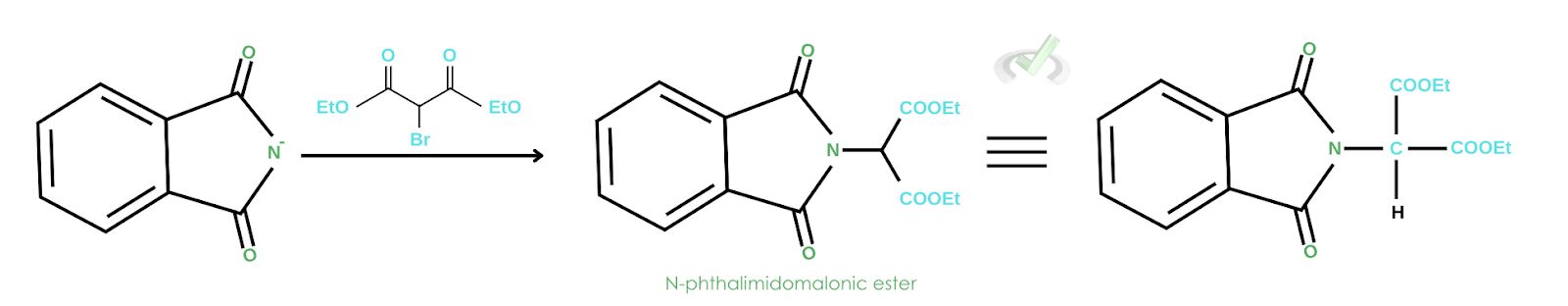
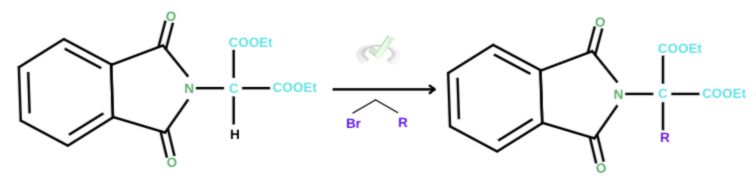
An SN2 reaction is simply a nucleophilic substitution reaction in the second order. It’s the same as the substitution reaction we’ve been talking about, which initiates the removal of a leaving group. In this instance, when a malonic ester is introduced, bromide gets ejected to give an N-phthalimidomalonic ester. Alkylation follows through the R-Br group. The carbon now acts as a nucleophile, attacks the R group, and ejects the bromide.
3. Acid hydrolysis
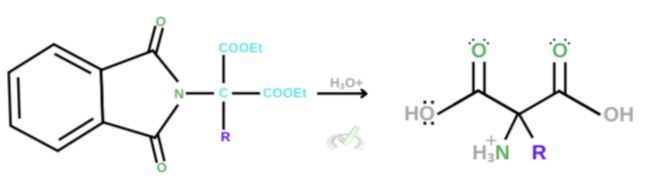
In this phase, many reactions occur. Acid hydrolysis happens in an acidic environment. As this happens, remember that in hydrolysis, we’re essentially breaking apart compounds and replacing them with an -OH group. This is what happens during acid hydrolysis. The carbonyl group on either side of the nitrogen breaks off its bonds with the benzene ring and is replaced by an OH group.
4. Decarboxylation
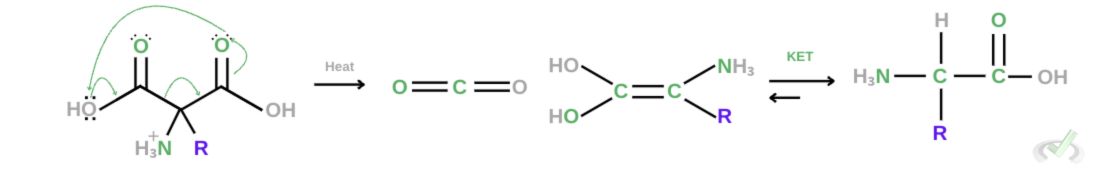
Picking up from where we left off, once we heat this up, electrons will rearrange to gain stability; this rearrangement will result in the release of carbon dioxide. Notice that this is still not an amino group since we still have an extra OH group on one side. We also see that this is an enol since it has a double bond with carbon and an alcohol group. This will then go through keto-enol tautomerization, which forms an amino acid.
III. Conclusion
Amino acid synthesis can be done in the laboratory using certain techniques. Two of which are Strecker synthesis and Gabriel synthesis. Strecker synthesis uses ammonium and cyanide to turn aldehydes into amino acids. The process involves imine formation, cyanide addition, and acid hydrolysis. This method makes racemic amino acids. Gabriel synthesis, on the other hand, requires deprotonation using a strong base, substitution reaction using an alkyl halide, acid hydrolysis, and decarboxylation through heating. This method is sometimes called Gabriel malonic ester synthesis due to the addition of a malonic ester in the process.
IV. Key Terms
- Enantiomer - A pair of molecules that are mirror images of each other with different means of interacting with other compounds.
- Chiral - A chiral molecule has at least one chiral center that has one carbon with four substituents.
- Gabriel Synthesis - A laboratory technique that uses alkyl halides and malonic ester to produce amino acids.
- Hydrolysis - A chemical reaction in which water breaks down a compound.
- Malonic ester - A compound with two ester groups joined together by a central malonic acid structure.
- Racemic - A racemic mixture contains equal parts of two enantiomers of a chiral molecule.
- Strecker Synthesis - A method that uses ammonium and cyanide to make amino acids.
V. Practice Questions
Sample Practice Question 1
All of the following statements are true except:
A. Gabriel synthesis involves making a primary amine for amino acids.
B. Strecker synthesis involves adding CN- to an imine.
C. Amino acids made through strecker synthesis are racemic.
D. Racemic mixtures have a 1:2 ratio of stable to unstable configurations of an amino acid.
Ans. D
Sample Practice Question 2
Which of the following would not explain why racemic amino acids are not quite favored over naturally occurring amino acids?
A. Some species are specific to L-configured amino acids since all animal amino acids have an L-configuration.
B. Amino acids with D-configuration are seen only in special structures, which could mean that a racemic mixture might contain inactive amino acids.
C. D-enantiomers participate in biological processes.
D. Further purification is needed to isolate L-enantiomers.
Ans. C







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
