I. What is Glycogenolysis?
Again, welcome back assuming you’re here from our glycogenesis article! If you’re starting to reverse your mentality from the previous article, you’re already on the right track! Always try to employ studying these “partner” topics side by side to make your MCAT prep more efficient!
Though it’s not simply the glycogenesis enzymes catalyzing the reverse reaction, glycogenolysis is also a relatively simple process with only a few enzymes to become familiar with! Try to put the glycogenesis and glycogenolysis enzymes to see which ones oppose each other.
As mentioned in the previous article, one of the best ways to review these 2 processes is to apply and connect their function to the body’s physiology. As a matter of fact, these are often ways that the MCAT will test these processes, so we’ll make sure to give y'all some practice!
II. Metabolism of Glycogen | Glycogenolysis
We’ve already covered the structure and function of glycogen in our previous article; feel free to look back to that article if you need a refresher before starting this article!
A. Glycogenolysis
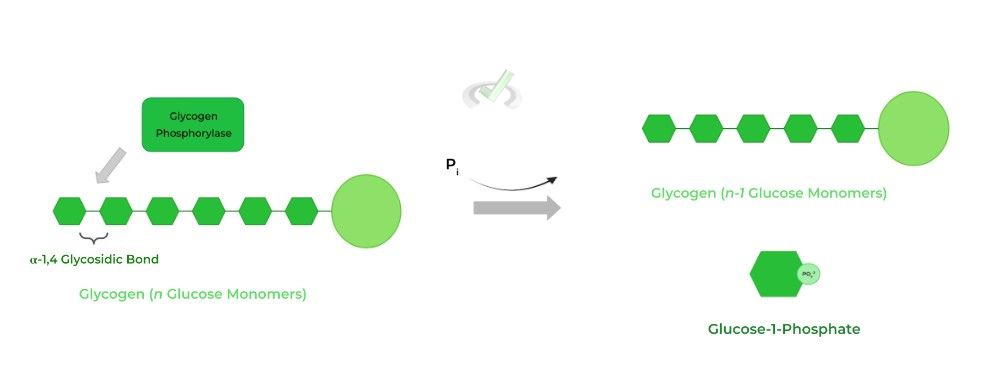
As such, glycogenolysis results in the breakdown of glycogen via the release of a glucose monomer! Recall that the release of glucose from glycogen must take place at the terminal end!
From a terminal end, the rate limiting enzyme glycogen phosphorylase hydrolyzes the first 𝛼-1,4 glycosidic bond releasing 1 glucose. The enzyme also catalyzes the attachment of a free phosphate group, regenerating glucose-1-phosphate.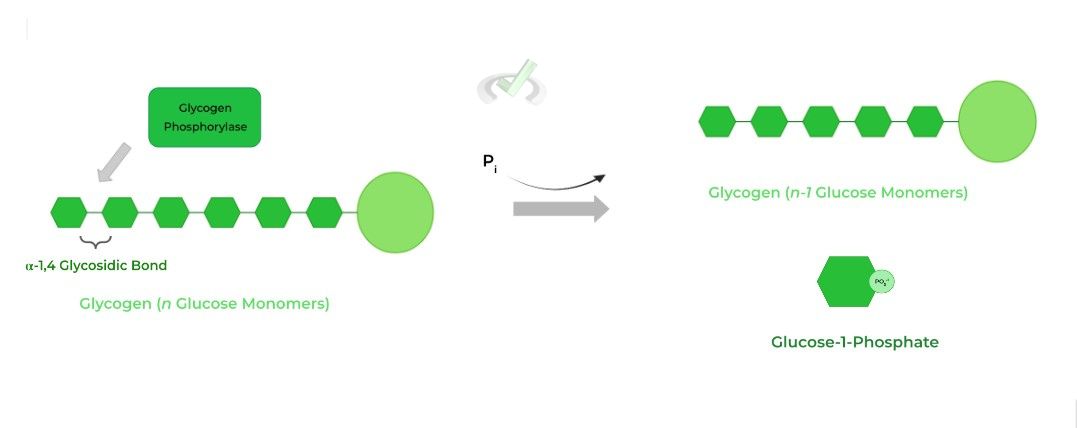
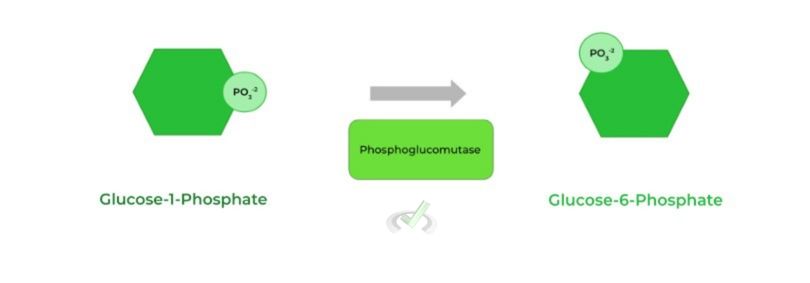
The glucose-1-phosphate molecule can then be isomerized back to glucose-6-phosphate which can then either undergo glycolysis or gluconeogenesis depending on the conditions.
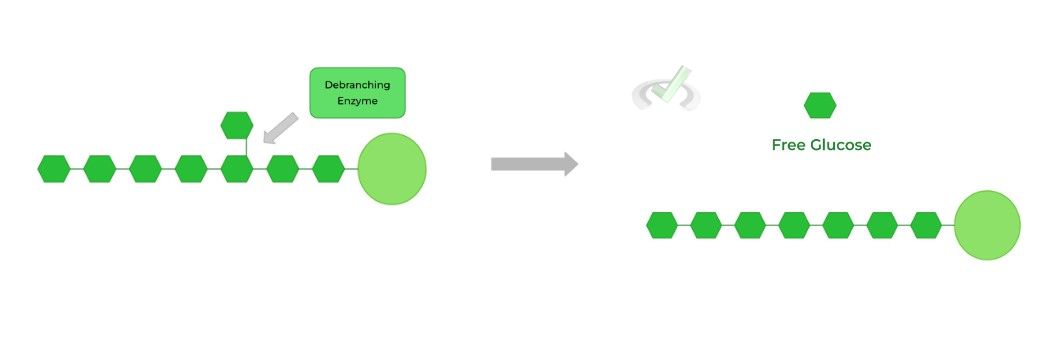
I. Debranching of Branched Chains
Because glycogen phosphorylase can only hydrolyze 𝛼-1,4 glycosidic bonds, this poses a problem for the last glucose on the branch as it’s connected to another linear chain via an 𝛼-1,6 glycosidic bond.
Here, the debranching enzyme helps to solve this! About 4 glucose residues before the branch point, the debranching enzyme hydrolyzes the 𝛼-1,4 bond of the branched glucose, releasing a short chain glycogen oligomer.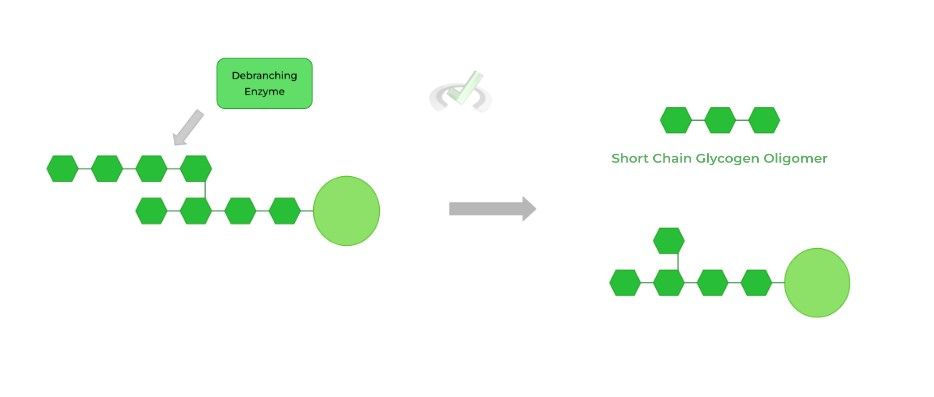
The debranching enzyme then repositions short chain glycogen oligomer to form a new 𝛼-1,4 glycosidic bond with a linear chain to extend it! This way, glycogen phosphorylase can now resume its activity.
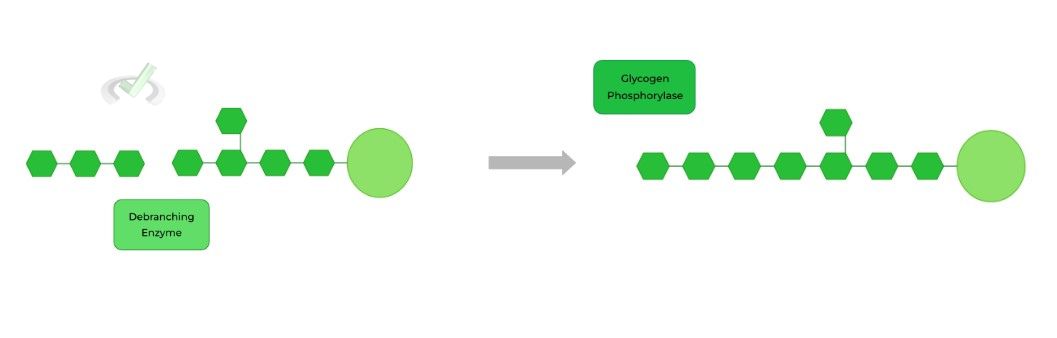
The debranching enzyme then hydrolyzes the 𝛼-1,6 glycosidic bond, releasing the last remaining branched glucose!
III. Bridge/Overlap
Need a refresher on the differences between a kinase and phosphorylase (like the one we saw earlier, glycogen phosphorylase)? We got you covered! Let’s throw it back a little bit for a quick review!
I. Kinase v.s. Phosphorylase
While both involve the covalent addition of a phosphate group to another molecule, the 2 enzymes differ in the SOURCE of the phosphate.
A kinase will utilize ATP as its phosphate source and will transfer the 𝛾-phosphate group to another molecule such as in the phosphorylation of an enzyme at a serine, threonine, and tyrosine residue, such as in hexokinase.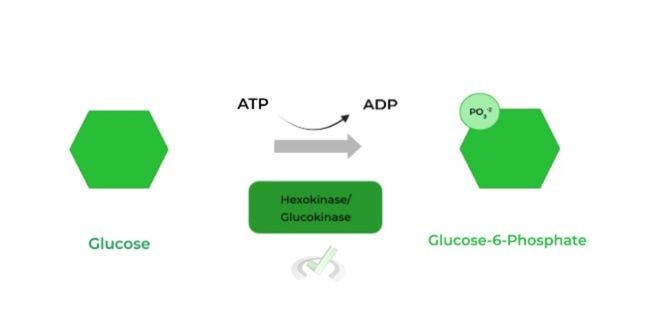
A phosphorylase will use a free phosphate group, not one already bound to a molecule (i.e. ATP )as its source. This is shown with glycogen phosphorylase as it attaches a free phosphate group to the released glucose, generating glucose-1-phosphate.
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Glycogenolysis
Refers to the breakdown of glycogen releasing a glucose monomer from the terminal end! Glycogen phosphorylase hydrolyzes the first 𝛼-1,4 glycosidic bond from the end to release a glucose molecule.
Glycogen phosphorylase also catalyzes the attachment of a free phosphate group to the released glucose to form glucose-1-phosphate, which can then be isomerized back to glucose-6-phosphate.I. Debranching of Branched Chains
Because glycogen phosphorylase can only hydrolyze the 𝛼-1,4 bonds, the debranching enzyme allows for the release of the branched glucose, as it’s connected via an 𝛼-1,6 bond.
When about 4 glucose residues are left on the branched chain, the debranching enzyme hydrolyzes the 𝛼-1,4 bond of the branched glucose, releasing a short chain glycogen oligomer.
The short chain oligomer is then repositioned to extend an existing linear chain by forming an 𝛼-1,4 bond catalyzed by the debranching enzyme.
The remaining branched glucose is then released by the hydrolysis of the 𝛼-1,6 bond also catalyzed by the debranching enzyme.
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1:
Which of the following has the ability to hydrolyze an 𝛼-1,4 glycosidic bonds?
I. Glycogen Synthase
II. Debranching Enzyme
III. Glycogen Phosphorylase
A. II only
B. III only
C. I and III
D. II and III
Ans. D
The debranching enzyme will hydrolyze an 𝛼-1,4 glycosidic bond to release the short chain oligomer and reposition it to extend an existing linear chain!
In addition, glycogen phosphorylase will hydrolyze an 𝛼-1,4 glycosidic bond to release a glucose from a terminal end and add a free phosphate group to generate glucose-1-phosphate.
Sample Practice Question 2:
After a period of fasting, which of the following enzymes is most likely to be INHIBITED within the body?
A. Glycogen Phosphorylase
B. Debranching Enzyme
C. Glycogen Synthase
D. Phosphoglucomutase
Ans. C
Be careful with the wording of the question! After a period of fasting, it would be expected that glycogen breakdown (glycogenolysis) would be INCREASED to release glucose that can be used for energy in the cell and/or to be released into the blood to increase blood glucose. As such, glycogen synthesis (glycogenesis) should DECREASE.
This being so, the rate limiting enzyme of glycogenesis, glycogen synthase should be the enzyme that’s inhibited after this period of fasting.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot