Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful analytical technique used in organic chemistry to determine the structure of molecules. NMR works by detecting the interaction of nuclear spins when placed in a magnetic field. Understanding NMR spectroscopy helps identify a molecule's different types of hydrogen and carbon atoms.
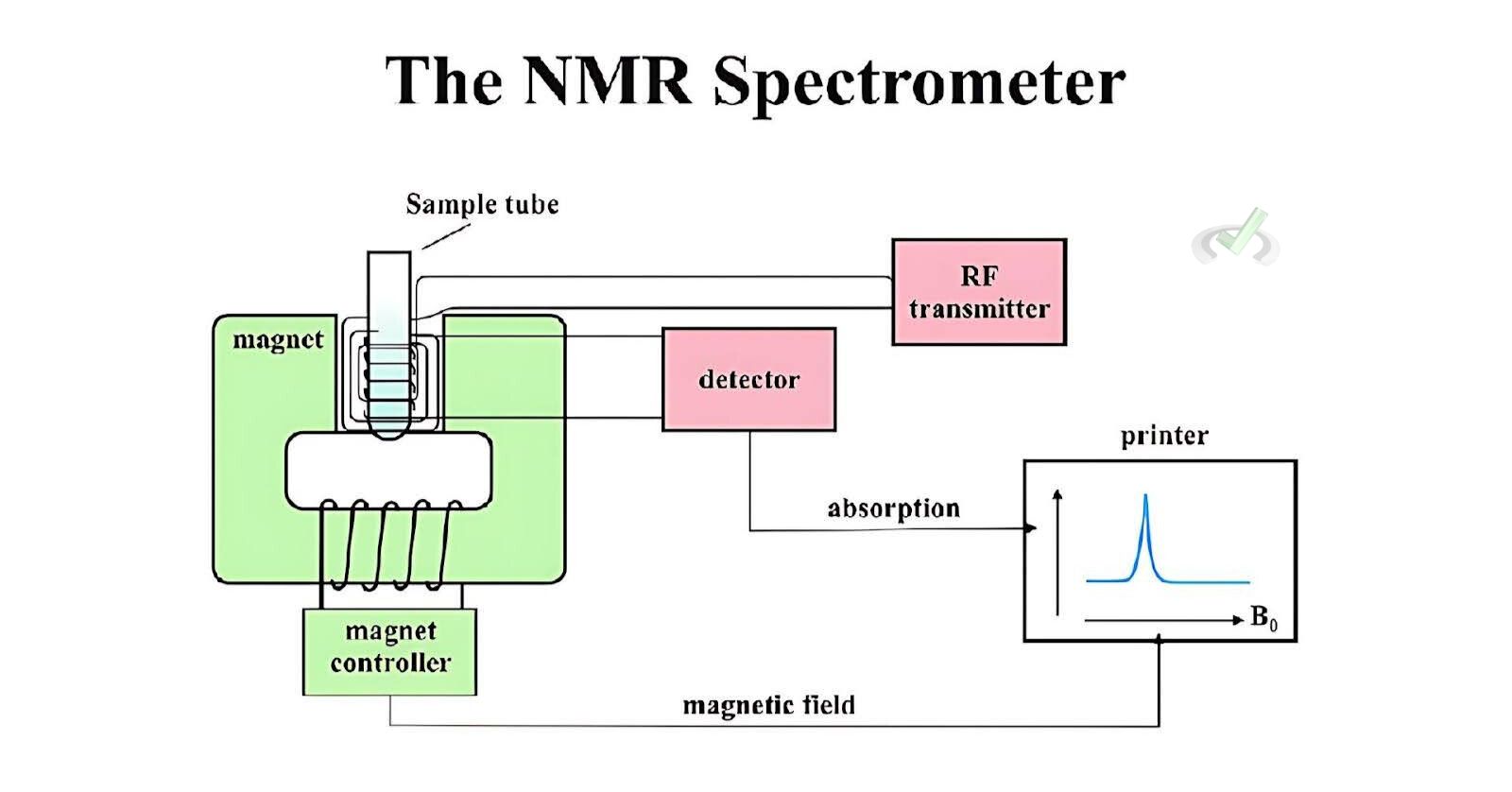
I. Basics of NMR Spectroscopy
NMR spectroscopy provides information about the environment of nuclei in a molecule. The most common types are proton NMR (¹H NMR) and Carbon-13 NMR (¹³C NMR).
A. Proton NMR (¹H NMR)
¹H NMR spectroscopy focuses on detecting hydrogen atoms in a molecule. Each hydrogen atom (proton) has a unique environment based on the atoms around it. This environment affects the proton's resonance frequency.
- Chemical Shift (δ): This is the position of an NMR signal relative to a standard reference. It is measured in parts per million (ppm). Different chemical environments cause protons to resonate at various frequencies.
- Integration: The area under an NMR signal indicates the number of protons contributing to that signal.
- Multiplicity (Splitting Patterns): Signals can split into multiple peaks due to interactions with neighboring protons. The splitting pattern (singlet, doublet, triplet, etc.) provides information about the number of adjacent protons.
B. Carbon-13 NMR (¹³C NMR)
¹³C NMR spectroscopy detects carbon atoms in a molecule. It is less sensitive than ¹H NMR but provides crucial structural information.
- Chemical Shift (δ): Carbon atoms in different environments resonate at different frequencies, measured in ppm.
- No Integration: Unlike ¹H NMR, the signal intensity in ¹³C NMR is not proportional to the number of carbons.
II. Understanding NMR Spectra
Interpreting NMR spectra involves analyzing chemical shifts, splitting patterns, and signal intensities. Let's look at some examples.
A. Example 1: Ethanol (C₂H₅OH)
¹H NMR Spectrum:
Chemical Shifts:
- CH₃ group: δ 1.2 ppm (triplet)
- CH₂ group: δ 3.6 ppm (quartet)
- OH group: δ 2-4 ppm (broad singlet, varies with hydrogen bonding)
Integration: The area ratio is 3:2:1 for CH₃, CH₂, and OH protons, respectively.
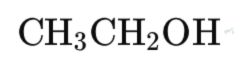
B. Example 2: Benzene (C₆H₆)
¹H NMR Spectrum:
Chemical Shifts:
Aromatic protons: δ 7.2 ppm (singlet)
Integration: All six protons are equivalent, so the area under the singlet corresponds to six protons.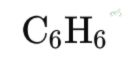
III. Important Concepts in NMR Spectroscopy
A. Shielding and Deshielding
Shielding: Protons or carbons surrounded by electron density are "shielded" and resonate at higher fields (lower δ).
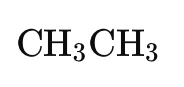
Deshielding: Protons or carbons near electronegative atoms or double bonds are "deshielded" and resonate at a lower field (higher δ).

B. Coupling Constants (J values)
Coupling constants measure the interaction between neighboring nuclei. They are reported in Hertz (Hz). Larger J values indicate stronger coupling.
IV. Applications of NMR Spectroscopy
NMR spectroscopy is used in various applications, from identifying unknown compounds to studying the dynamics of molecules. Here are some critical applications:
A. Structure Elucidation
NMR helps determine the structure of organic compounds by providing detailed information about the carbon and hydrogen framework. By analyzing the chemical shifts, splitting patterns, and coupling constants, chemists can deduce the arrangement of atoms in a molecule.
Example:
Ethanol (C₂H₅OH): The ¹H NMR spectrum of ethanol shows distinct signals for the CH₃, CH₂, and OH groups. The chemical shifts and splitting patterns help identify these groups' positions in the molecule.

B. Studying Chemical Reactions
NMR can monitor reaction progress and identify intermediates and products. By taking NMR spectra at different time points, chemists can observe changes in the chemical environment of nuclei and determine which species are present at each reaction stage.
Example:
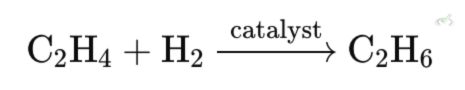
Hydrogenation of Alkenes: NMR can be used to monitor the hydrogenation of ethene (C₂H₄) to ethane (C₂H₆). The disappearance and appearance of the alkene signal indicate the reaction's progress.
C. Biological Applications
NMR is used to study proteins, nucleic acids, and other biomolecules. It provides information about the three-dimensional structure, dynamics, and interactions of these molecules, which is crucial for understanding biological functions and designing drugs.
Example:
In protein structure determination, NMR can determine the structure of small proteins in solution. Researchers can determine the distances between atoms by analyzing the NOE (Nuclear Overhauser Effect) signals and building a three-dimensional protein model.
V. Advanced Topics in NMR Spectroscopy
Here are some advanced topics:
A. 2D NMR Spectroscopy
Two-dimensional (2D) NMR spectroscopy offers a more comprehensive understanding of molecular structures. It achieves this by distributing the data across two frequency dimensions.
This enhances the resolution and facilitates the differentiation of overlapping signals. As a result, it enables more detailed and accurate identification of molecular properties.
- COSY (Correlation Spectroscopy): COSY helps determine which protons are coupled to each other. This technique shows correlations between protons close to each other in the molecule, revealing connectivity between hydrogen atoms.
- HSQC (Heteronuclear Single Quantum Coherence): HSQC correlates hydrogen atoms with the carbon atoms to which they are directly bonded. This technique helps identify which hydrogen atoms are attached to which carbon atoms in the molecule.
B. NOE (Nuclear Overhauser Effect)
The Nuclear Overhauser Effect (NOE) provides information about the spatial proximity of atoms within a molecule. It arises from the transfer of nuclear spin polarization from one nucleus to another through space.
NOE measurements help determine the distances between atoms that are close in space but not necessarily bonded. This is particularly useful in studying the three-dimensional structures of large molecules like proteins.
C. Dynamic NMR
Dynamic NMR is employed to analyze molecules that undergo rapid conformational changes. This method helps determine the rates of chemical processes and the energy barriers between various conformations. By monitoring how NMR signals shift with temperature changes, researchers can more effectively understand molecules' flexibility and dynamics.
VI. Wrap-Up and Key Terms
Understanding NMR spectroscopy involves mastering several key concepts and principles. Let's review:
- Chemical Shift (δ): Position of an NMR signal relative to a standard reference, measured in ppm.
- Integration: Area under an NMR signal, indicating the number of protons.
- Multiplicity (Splitting Patterns): Pattern of peaks due to interactions with neighboring protons.
- Shielding and Deshielding: Effects of electron density on resonance frequency.
- Coupling Constants (J values): Measure interaction between neighboring nuclei, reported in Hz.
- COSY: Correlation spectroscopy is used to identify coupling between protons.
- HSQC: Correlates hydrogen and carbon atoms directly bonded to each other.
- NOE: Provides information about the spatial proximity of atoms within a molecule.
VII. Practice Questions
Sample Practice Question 1
What does the integration of a signal in an NMR spectrum indicate?
A) The chemical environment of the protons
B) The number of protons contributing to the signal
C) The coupling constant
D) The chemical shift
Ans. B
Integrating a signal in an NMR spectrum indicates the number of protons contributing to that signal.
Sample Practice Question 2
In a ¹H NMR spectrum, what does a quartet signal typically indicate?
A) The presence of four neighboring protons
B) The presence of three neighboring protons
C) The presence of two neighboring protons
D) The presence of one neighboring proton
Ans. B
A quartet signal in a ¹H NMR spectrum typically indicates the presence of three neighboring protons, as splitting follows the n+1 rule.



 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
























