I. What are Biological Oxidation-Reduction Reactions?
Hopefully from the name itself, you can already see the possible overlaps and bridges that can be made between this general chemistry and biochemistry! Interestingly enough, a lot of the cellular, biochemical processes involve the transfer of electrons!
Whether it’s the digestion of glucose or the production of precursors to nucleic acids, all the processes have the single commonality of having some form of electron transfer occurring, with some to a much greater extent than others!
Understanding and reviewing this section will probably be most beneficial when put in the context of carbohydrate metabolism! But, as always, look at the many other biochemical reactions that can also be applicable when reviewing this section!
II. Electron Transfer in Biochemical Reactions
Even when placed in a biochemical, cellular context, the basic principles of redox reactions remain the same! Let’s first get into a basic review of redox reactions and identify some key electron carriers!
A. Half Reactions
Recall that oxidation refers to the loss of electrons while reduction refers to the gain of electrons, simply put via the “OIL-RIG” mnemonic.
Similarly, a biochemical reaction can be broken down into 2 half reactions: one for the oxidation reaction and another for reduction.
Take a look at the example below of the reaction that occurs in complex I (NADH dehydrogenase) of the ETC!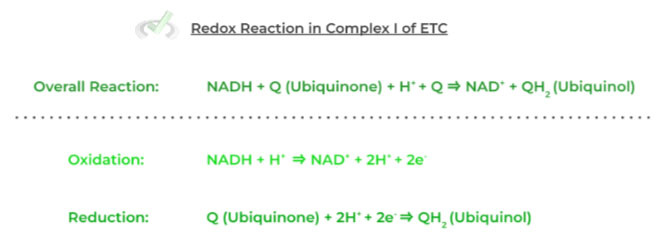
Remember always to make sure everything adds up as well as cancels out correctly to follow conservation of mass and charge!
Notice how everything balances out, as the number of electrons lost in the oxidation reaction is the same number of electrons gained in the reduction half reaction.
B. Key Electron Carriers
In many biochemical reactions, the reduction half reaction usually results in the production of high energy electron carriers.
Probably the most well known example of their use is in the electron transport chain. Let’s take a look at a couple of them!
I. NADH: Nicotinamide Adenine Dinucleotide
This is probably the most common electron carrier you’ll encounter, as it’s produced in many of the carbohydrate metabolic pathways including glycolysis and the citric acid cycle.
After it’s produced, NADH will transfer its electrons to complex I (NADH dehydrogenase), which ultimately powers the production of ATP synthesis.
II. FADH2: Flavin Adenine Dinucleotide
Similarly, this electron carrier is also produced in various metabolic reactions including the citric acid cycle.
In the context of the ETC, FADH2 will donate its electron to complex II (succinate dehydrogenase), also indirectly powering ATP synthesis.
There are various other electron carriers, but these are the ones you’ll definitely encounter the most during your MCAT prep, especially during carbohydrate metabolism!
III. Bridge/Overlap
In the half reaction shown above, you may come to one why isn’t it the other way around. Why is it that ubiquinone will be reduced to ubiquinol and NADH will be oxidized back to NAD+? This all comes back to the concept of standard reduction potential!
I. Standard Reduction Potential
Recall that standard reduction potential is just a value given to a molecule which tells how likely it is to be reduced. The more positive the value is, the more likely the molecule is to be reduced!
Let’s look at the above example of NADH and ubiquinone to get a better idea. Shown below are the 2 reactions if a molecule was being reduced.
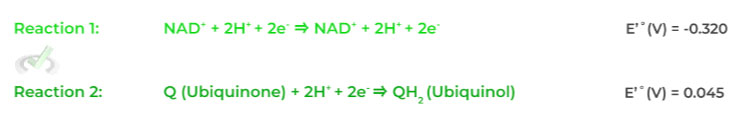
Notice how the reduction of ubiquinone to ubiquinol has a more positive standard reduction potential than that of NAD+ to NADH, which results in its reduction during complex I.
Think of it like they’re taking turns! NAD+ was reduced to NADH in glycolysis and the citric acid cycle. But now because ubiquinone has a higher standard reduction potential, it’s now its turn to get reduced in complex I.
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Half Reactions
We can split a biochemical reaction into 2 half reactions: one for the oxidation (loss of electrons) reaction and another for the reduction (gain of electrons) reaction.
Be careful and sure to balance out everything! As the law of conservation of mass and charge states, the amount of electrons lost in oxidation should equal the amount of electrons gained in reduction.B. Key Electron Carriers
Many of the biochemical reactions and metabolic pathways will produce high energy electron carriers, which are used in many reactions, but are most significant in the electron transport chain.
I. NADH: Nicotinamide Adenine Dinucleotide
Recall that this molecule is produced in many carbohydrate metabolic reactions including glycolysis, the pyruvate dehydrogenase complex, and the citric acid cycle.
Its role as an electron carrier is seen in complex I (NADH dehydrogenase) where it transfers its electrons to power ATP synthesis.II. FADH2: Flavin Adenine Dinucleotide
Recall that this molecule is also produced in the citric acid cycle! Similarly, it transfers its electrons to complex II (succinate dehydrogenase) to also indirectly power ATP synthesis.
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
Given the overall redox reaction shown and its following half reactions shown below, which reaction is the oxidation reaction?
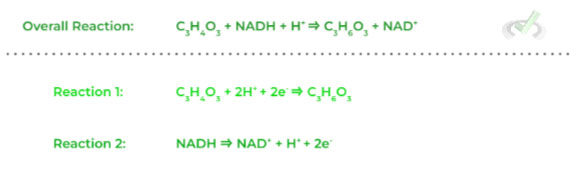
A. Reaction 1 because it loses electrons
B. Reaction 1 because it gains electrons
C. Reaction 2 because it loses electrons
D. Reaction 2 because it gains electrons.
Ans. C
To solve this question, simply look at which reaction is losing electrons as free electrons should be on the product side of the half reaction equation as seen in reaction 2.
Sample Practice Question 2:
In the context of the electron transport chain, which of the following has the highest standard reduction potential?
A. NAD+
B. Ubiquinone
C. Cytochrome C
D. Oxygen
Ans. D
Recall that standard reduction potential is the value which shows how likely a molecule is to undergo reduction. It makes sense that oxygen has the highest standard reduction potential because it’s the last electron acceptor in the ETC!







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot